Tooth Whitening with Hydroxyapatite: A Systematic Review
Abstract
:1. Introduction
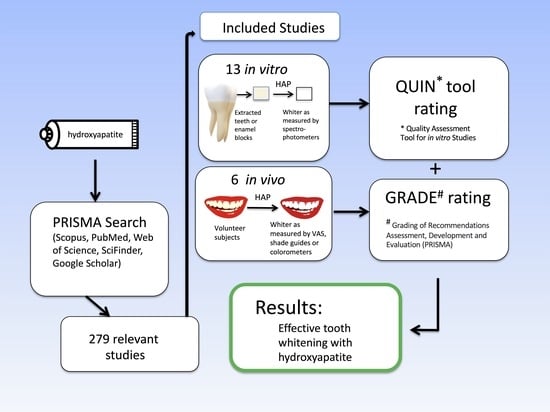
2. Materials and Methods
3. Results
| Study (Country) [Reference] | Type of Study | Clinical Study Details | Tested Materials and Products | Conclusion of the Publication Authors | GRADE Assignment of HAP Whitening Effect Conclusion of the Authors of This Review |
|---|---|---|---|---|---|
| Steinert, Kuchenbecker et al., 2020 (Germany) [50] | in vivo | Twenty-five subjects; subjective change in tooth color after 4 weeks of use; -questionnaire; -no control, no blinding. | HAP gel. | “In conclusion, microcrystalline hydroxyapatite is a promising whitening agent for oral care formulations and represents a biomimetic alternative to other whitening agents for daily dental care”. | LOW subjective HAP-whitening effect reported. |
| Steinert, Zwanzig et al., 2020 (Germany) [51] | in vivo | Forty-six subjects; -28 days of trial brushing at home; -VAS scale for color change; -no control (before and after design); -questionnaire results. | Toothpaste with 20% biomimetic zinc HAP. | “Additionally, patients reported smoother and whiter teeth after using the HAP toothpaste”. | LOW HAP-whitening effect confirmed. |
| Bommer et al., 2018 (Switzerland, Germany) [42] | in vivo | -Forty subjects; -1 month; -no treatment and placebo controls; -whitening index was used to measure the effect. | Mixture of self-assembling peptide matrix and HAP. | “The combination of SAPM+HA particles caused optical whitening based on diffuse reflection by the HA particles on the tooth surface. The whitening effect and its magnitude observed in vitro were also seen in vivo”. | MODERATE HAP-whitening effect confirmed. |
| Woo et al., 2014 (Korea) [52] | in vivo | -Eighty-five subjects; -3 months of trial; -VAS scale of whitening; -double-blinded, randomized. | Toothpastes containing 0.75% hydrogen peroxide, 0.25% HAP, and placebo. | “The hydrogen peroxide-containing toothpaste caused significant lightening of tooth coloration, (mopre) than the hydroxyapatite and placebo toothpastes”. | LOW -only a very small amount of HAP was used (0.25% HAP), other studies tested concentrations up to 20-30% HAP; -a higher concentration of hydrogen peroxide (0.75%) was used as the control than is allowed in cosmetic toothpastes in the EU (0.1%). |
| Raoufi and Birkhed 2010 (Sweden) [53] | in vivo | -One-hundred and fifty subjects; -12 weeks of study; -Vita Easy shade test; -double-blinded, randomized. | Toothpastes containing HAP, calcium peroxide, and placebo. | “The toothpaste containing hydroxyapatite or calcium peroxide did not produce any reduction in tooth staining compared with a placebo fluoride toothpaste”. | LOW -only a very small amount of HAP was used (0.1% HAP or less; no exact amount was given); other studies tested concentrations up to 20–30% HAP; -interestingly, even the peroxide group was not superior to a control toothpaste. |
| Niwa et al., 2001 (Japan) [49] | in vivo | -Twelve subjects; -4 weeks of trial; -colorimeter with fiberscope to measure brightness and whiteness; -five volunteers in the 3% HAP group, seven volunteers in the 15% HAP group (no details of blinding, randomization). | Toothpastes containing 3% and 15% HAP were compared to the placebo (no HAP). | “It is concluded that toothpaste containing hydroxyapatite are effective at whitening tooth and that whitening was not due to their polishing effect on tooth surface”. | LOW HAP-whitening effect confirmed (15% HAP more efficient that 3% HAP). |
4. Discussion
4.1. Abrasivity
4.2. Peroxide Side Effects
4.3. Mechanism of Whitening with HAP Toothpaste
4.3.1. Immediate Whitening Effect
4.3.2. Long-Term Whitening
4.4. Effects of Increasing Concentration of HAP in Toothpaste
4.5. Limitations of the Review
4.6. The Future of Hydroxyapatite Toothpastes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aida, J.; Takeuchi, K.; Furuta, M.; Ito, K.; Kabasawa, Y.; Tsakos, G. Burden of oral diseases and access to oral care in an ageing society. Int. Dent. J. 2022, 72, S5–S11. [Google Scholar] [CrossRef]
- Newton, J.T.; Subramanian, S.S.; Westland, S.; Gupta, A.K.; Luo, W.; Joiner, A. The impact of tooth colour on the perceptions of age and social judgements. J. Dent. 2021, 112, 103771. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Quiñonez, C. Straight, white teeth as a social prerogative. Sociol. Health Illn. 2015, 37, 782–796. [Google Scholar] [CrossRef]
- Chadwick, B.; Cage, B.; Playle, R. Do teenage magazines give a genuine view of tooth colour? Br. Dent. J. 2007, 203, E9. [Google Scholar] [CrossRef] [PubMed]
- Grumsen, S. The era of whiter teeth: Advertising in American dentistry 1910–1950. J. Hist. Dent. 2009, 57, 75–84. [Google Scholar]
- Carey, C.M. Tooth whitening: What we now know. J. Evid. Based Dent. Pract. 2014, 14, S70–S76. [Google Scholar] [CrossRef]
- Gómez Polo, C.; Gómez Polo, M.; Montero, J.; Martínez Vazquez De Parga, J.A.; Celemin Viñuela, A. Correlation of natural tooth colour with aging in the Spanish population. Int. Dent. J. 2015, 65, 227–234. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Scully, C.; Bagan-Sebastian, J.V. Drug-induced disorders of teeth. J. Dent. Res. 2005, 84, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Suprono, M.; Mateo, L.R.; Zhang, Y.P.; Denis, J.; D’Ambrogio, R.; Sullivan, R.; Thomson, P. Solving the problem with stannous fluoride: Extrinsic stain. J. Am. Dent. Assoc. 2019, 150, S38–S46. [Google Scholar] [CrossRef]
- Frese, C.; Wohlrab, T.; Sheng, L.; Kieser, M.; Krisam, J.; Wolff, D. Clinical effect of stannous fluoride and amine fluoride containing oral hygiene products: A 4-year randomized controlled pilot study. Sci. Rep. 2019, 9, 7681. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciù, M. Stannous fluoride effects on enamel: A systematic review. Biomimetics 2020, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A. Whitening toothpastes: A review of the literature. J. Dent. 2010, 38, e17–e24. [Google Scholar] [CrossRef]
- Theilade, J.; Pang, K.M. Scanning electron microscopy of black stain on human permanent teeth. Scanning Microsc. 1987, 1, 1983–1989. [Google Scholar]
- Gasmi Benahmed, A.; Gasmi, A.; Menzel, A.; Hrynovets, I.; Chirumbolo, S.; Shanaida, M.; Lysiuk, R.; Shanaida, Y.; Dadar, M.; Bjørklund, G. A review on natural teeth whitening. J. Oral. Biosci. 2022, 64, 49–58. [Google Scholar] [CrossRef]
- Epple, M.; Meyer, F.; Enax, J. A critical review of modern concepts for teeth whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef]
- Haywood, V.B. Bleaching of vital and nonvital teeth. Curr. Opin. Dent. 1992, 2, 142–149. [Google Scholar] [PubMed]
- Maran, B.M.; Burey, A.; de Paris Matos, T.; Loguercio, A.D.; Reis, A. In-office dental bleaching with light vs. without light: A systematic review and meta-analysis. J. Dent. 2018, 70, 1–13. [Google Scholar] [CrossRef]
- Goldberg, M.; Grootveld, M.; Lynch, E. Undesirable and adverse effects of tooth-whitening products: A review. Clin. Oral. Investig. 2010, 14, 1–10. [Google Scholar] [CrossRef]
- Kim, D.H.; Bae, J.; Heo, J.H.; Park, C.H.; Kim, E.B.; Lee, J.H. Nanoparticles as next-generation tooth-whitening agents: Progress and perspectives. ACS Nano 2022, 16, 10042–10065. [Google Scholar] [CrossRef]
- Casado, B.G.S.; Moraes, S.L.D.; Souza, G.F.M.; Guerra, C.M.F.; Souto-Maior, J.R.; Lemos, C.A.A.; Vasconcelos, B.C.E.; Pellizzer, E.P. Efficacy of dental bleaching with whitening dentifrices: A systematic review. Int. J. Dent. 2018, 2018, 7868531. [Google Scholar] [CrossRef]
- Collins, L.Z.; Naeeni, M.; Platten, S.M. Instant tooth whitening from a silica toothpaste containing blue covarine. J. Dent. 2008, 36, S21–S25. [Google Scholar] [CrossRef]
- Vaz, V.T.P.; Jubilato, D.P.; Oliveira, M.R.M.; Bortolatto, J.F.; Floros, M.C.; Dantas, A.A.R.; Oliveira Junior, O.B. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: Which one is the most effective? J. Appl. Oral Sci. 2019, 27, e20180051. [Google Scholar] [CrossRef]
- Schemehorn, B.R.; Moore, M.H.; Putt, M.S. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J. Clin. Dent. 2011, 22, 11–18. [Google Scholar]
- Coceska, E.; Gjorgievska, E.; Coleman, N.J.; Gabric, D.; Slipper, I.J.; Stevanovic, M.; Nicholson, J.W. Enamel alteration following tooth bleaching and remineralization. J. Microsc. 2016, 262, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Naik, S.; Lewis, N.J.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef]
- Redha, O.; Mazinanian, M.; Nguyen, S.; Son, D.O.; Lodyga, M.; Hinz, B.; Odlyha, M.; McDonald, A.; Bozec, L. Compromised dental cells viability following teeth-whitening exposure. Sci. Rep. 2021, 11, 15547. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.D.; Han, J.M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Clinical evidence of hydroxyapatite in oral care products for reducing dentin hypersensitivity: An updated systematic review and meta-analysis. Biomimetics 2023, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products-A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Licensed Natural Health Products. Available online: https://health-products.canada.ca/lnhpd-bdpsnh/info.do?licence=80117093 (accessed on 5 January 2023).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2022, 22, S00345–S00346. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Santesso, N.; Brozek, J.; Akl, E.A.; Walter, S.D.; Norman, G.; Kulasegaram, M.; Christensen, R.; Guyatt, G.H.; Falck-Ytter, Y.; et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J. Clin. Epidemiol. 2013, 66, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Hojabri, N.; Kunzelmann, K.H. Adhesion and whitening efficacy of P11-4 self-assembling peptide and HAP suspension after using NaOCl as a pre-treatment agent. BMC Oral Health 2022, 22, 59. [Google Scholar] [CrossRef]
- Shang, R.; Kaisarly, D.; Kunzelmann, K.H. Tooth whitening with an experimental toothpaste containing hydroxyapatite nanoparticles. BMC Oral Health 2022, 22, 331. [Google Scholar] [CrossRef]
- Shang, R.; Kunzelmann, K.H. Biomimetic tooth-whitening effect of hydroxyapatite-containing mouthrinses after long-term simulated oral rinsing. Am. J. Dent. 2021, 34, 307–312. [Google Scholar]
- Hojabri, N.; Kaisarly, D.; Kunzelmann, K.H. Adhesion and whitening effects of P11-4 self-assembling peptide and HAP suspension on bovine enamel. Clin. Oral Investig. 2021, 25, 3237–3247. [Google Scholar] [CrossRef]
- Sarembe, S.; Enax, J.; Morawietz, M.; Kiesow, A.; Meyer, F. In vitro whitening effect of a hydroxyapatite-based oral care gel. Eur. J. Dent. 2020, 14, 335–341. [Google Scholar] [CrossRef]
- Bommer, C.; Flessa, H.P.; Xu, X.; Kunzelmann, K.H. Hydroxyapatite and self-assembling peptide matrix for non-oxidizing tooth whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar] [PubMed]
- Jin, J.; Xu, X.; Lai, G.; Kunzelmann, K.H. Efficacy of tooth whitening with different calcium phosphate-based formulations. Eur. J. Oral Sci. 2013, 121, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, H.K.; Kim, B.I. Effect of nano-carbonate apatite to prevent re-stain after dental bleaching in vitro. J. Dent. 2011, 39, 636–642. [Google Scholar] [CrossRef]
- Dabanoglu, A.; Wood, C.; García-Godoy, F.; Kunzelmann, K.H. Whitening effect and morphological evaluation of hydroxyapatite materials. Am. J. Dent. 2009, 22, 23–29. [Google Scholar]
- Park, Y.D.; Kim, J.H.; Whang, K.S. Research about tooth whitening and bacteria sticking capability with using dentifrice including nano-hydroxyapatite, sodium metaphosphate. Key Eng. Mater. 2007, 330–332, 283–286. [Google Scholar]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309–311, 541–544. [Google Scholar]
- Park, Y.D.; Kambara, M.; Choi, E.; Hwang, K.S.; Kim, B.I. Research about changes of abrasiveness and whiteness of each dentifrice including nano-hydroxyapatite, sodium metaphosphate. Key Eng. Mater. 2006, 309–311, 545–548. [Google Scholar] [CrossRef]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening effects of a novel oral care gel with biomimetic hydroxyapatite: A 4-week observational pilot study. Biomimetics 2020, 5, 65. [Google Scholar] [CrossRef]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Woo, G.-J.; Kim, E.-K.; Jeong, S.-H.; Song, K.-B.; Goo, H.-J.; Jeon, E.-S.; Choi, Y.-H. Comparison of the whitening effect of toothpastes containing 0.25% hydroxyapatite and 0.75% hydrogen peroxide. J. Korean Acad. Oral Health 2014, 38, 3–9. [Google Scholar] [CrossRef]
- Raoufi, S.; Birkhed, D. Effect of whitening toothpastes on tooth staining using two different colour-measuring devices-a 12-week clinical trial. Int. Dent. J. 2010, 60, 419–423. [Google Scholar] [PubMed]
- Miskell, P. Cavity protection or cosmetic perfection? Innovation and marketing of toothpaste brands in the United States and Western Europe, 1955–1985. Bus. Hist. Rev. 2004, 78, 29–60. [Google Scholar] [CrossRef]
- Alshara, S.; Lippert, F.; Eckert, G.J.; Hara, A.T. Effectiveness and mode of action of whitening dentifrices on enamel extrinsic stains. Clin. Oral Investig. 2014, 18, 563–569. [Google Scholar] [CrossRef]
- González-Cabezas, C.; Hara, A.T.; Hefferren, J.; Lippert, F. Abrasivity testing of dentifrices—Challenges and current state of the art. Monogr. Oral Sci. 2013, 23, 100–107. [Google Scholar]
- American Dental Association. Available online: https://www.ada.org/resources/research/science-and-research-institute/oral-health-topics/toothpastes (accessed on 15 November 2022).
- Johannsen, G.; Tellefsen, G.; Johannsen, A.; Liljeborg, A. The importance of measuring toothpaste abrasivity in both a quantitative and qualitative way. Acta Odontol. Scand. 2013, 71, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Assunção, C.M.; Schlueter, N.; Rodrigues, J.A.; Carvalho, T.S.; Lussi, A. Do fluoride toothpastes have similar preventive effect in permanent and primary teeth against erosive tooth wear? Int. J. Paediatr. Dent. 2018, 29, 228–236. [Google Scholar] [CrossRef]
- Wiegand, A.; Kuhn, M.; Sener, B.; Roos, M.; Attin, T. Abrasion of eroded dentin caused by toothpaste slurries of different abrasivity and toothbrushes of different filament diameter. J. Dent. 2009, 37, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, A.; Schlueter, N. The role of oral hygiene: Does toothbrushing harm? Monogr. Oral Sci. 2014, 25, 215–219. [Google Scholar] [PubMed]
- Browning, W.D.; Cho, S.D.; Deschepper, E.J. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J. Esthet. Restor. Dent. 2012, 24, 268–276. [Google Scholar] [CrossRef]
- Gümüştaş, B.; Dikmen, B. Effectiveness of remineralization agents on the prevention of dental bleaching induced sensitivity: A randomized clinical trial. Int. J. Dent. Hyg. 2021, 20, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Fabritius-Vilpoux, K.; Enax, J.; Herbig, M.; Raabe, D.; Fabritius, H.-O. Quantitative affinity parameters of synthetic hydroxyapatite and enamel surfaces in vitro. Bioinspired Biomim. Nanobiomater. 2019, 8, 141–153. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Luong, M.N.; Gelfond, J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ Open 2021, 7, 23. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]


| Study (Country) [Reference] | Condition | Tested Materials/Products | Experimental Conditions and Results | Quoted Conclusion of the Publication | % RoB Scores * Strength of the Finding of HAP Whitening Effect |
|---|---|---|---|---|---|
| Hojabri and Kunzelmann 2022 (Germany) [37] | in vitro | Mixture of HAP (6.25%) and P11-4 self-assembling peptide (Curodont Repair). | Fifty bovine polished incisors treated with bleach immersed for 30 s in test and control pastes; -color changes measured by spectrophotometry; -color change ∆E = 1.27 ± 1.32; -p = 0.007 in contrast to water control. | “Pre-treatment with a low-concentrated NaOCl enhanced the adherence of the HAP layer on the enamel surface, resulting in a stronger whitening Effect”. | 79.2% HAP-whitening effect confirmed. |
| Shang et al. 2022 (Germany) [38] | in vitro | Toothpastes with 1% and 10% HAP. | Forty bovine incisor crowns polished, then stained in the lab; -toothpaste slurries applied with a toothbrush, then agitated, and color changes measured by spectrophotometry; - color change ∆E = 4.47; p < 0.05. | “nano-HAP toothpaste has a satisfying post brushing whitening effect and good resistance to mechanical forces. The whitening effect seemed to be concentration-dependent”. | 79.2% HAP-whitening effect confirmed (10% HAP was more efficient than 1% HAP). |
| Shang and Kunzelmann 2021 (Germany) [39] | in vitro | HAP (3 µm, 200 nm, and 50 nm particle size), commercial whitening mouth rinse, distilled water. | Fifty bovine incisor crowns polished, then stained in the lab; -exposure simulated for 3–6 months of mouth rinsing; -color changes measured by spectrophotometry; -color change ∆E = 3.07; p < 0.01. | “The HAP nanoparticles showed better tooth-whitening performance after a longer period of mouthrinsing than the microsized HAP particles”. | 79.2% HAP-whitening effect confirmed. |
| Hojabri et al. 2021 (Germany) [40] | in vitro | Experimental peptide-HAP suspensions (0.5% HAP, 6.25% HAP), commercial bleaching agent. | Forty bovine incisor crowns polished, then stained in the lab; -immersion in test mixtures for different times, then colorimetric color change; -color change ∆E = 6.42; -significant difference. | “The peptide-HAP suspension is a mild tooth whitener, and the adhesion of peptide-HAP to enamel is concentration dependent”. | 79.2% HAP-whitening effect confirmed. |
| Sarembe et al. 2020 (Germany) [41] | in vitro | Fifteen percent of HAP gel (Karex Gelée), whitening mouth rinse with phosphates, distilled water. | Twenty-eight bovine cleaned incisors immersed in test paste/rinse of 9 cycles; -color changes measured by spectrophotometry; -color change ∆E = 11.2 [±3.11]; p < 0.0001. | “This in vitro study demonstrated a significantly higher ad hoc whitening effect of the HAP gel compared to the mouth rinse and water after short-time application”. | 75% HAP-whitening effect confirmed. |
| Bommer et al. 2018 (Switzerland, Germany) [42] | in vitro | Mixture of self-assembling peptide matrix and 12.5% HAP. | Twenty stained bovine incisors; -micro brush application of mixture for 5 min, rinsed, and color changes measured by a colorimeter; -color change ∆E = 4.8 [±3.6]; p < 0.002. | “The combination of SAPM+HA particles caused optical whitening based on diffuse reflection by the HA particles on the tooth surface. The whitening effect and its magnitude observed in vitro were also seen in vivo”. | 71% HAP-whitening effect confirmed. |
| Jin et al. 2013 (Germany) [43] | in vitro | Toothpastes containing 10%, 20%, and 30% HAP; controls: A commercial toothpaste, a topical fluoride agent. | Ninety extracted caries-free polished human teeth; -3 min of application using a cotton pellet, allowed to sit for 5 min, agitated or stored, and color changes measured by a colorimeter; -color change ∆E = 0.91 [±0.50]; p < 0.05. | “Calcium phosphate-based formulations that can adhere to the enamel surface and contribute to tooth whitening have promising tooth-whitening potential”. | 79.2% HAP-whitening effect confirmed (30% Zn-HAP more efficient than both 20% Zn-HAP and 10% Zn-HAP). |
| Kim et al. 2011 (South Korea) [44] | in vitro | Ten percent of nano-carbonate apatite, casein phosphopeptide-amorphous calcium phosphate, NaF, distilled and deionized water. | Twenty-four bovine bleached incisors; -3-min treatments, four times per day, with test and control pastes, then pH cycled in artificial saliva to remineralize; -color change ∆E = 5.26 [±2.28]; p < 0.05. | “10% nano-carbonate apatite could significantly maintain the initial color and protect the damaged enamel structure after bleaching”. | 79.2% HAP-whitening effect confirmed. |
| Dabanoglu et al. 2009 (Germany) [45] | in vitro | Three HAP suspensions and two HAP mixtures in dissolvable polymer films. | Thirty extracted human premolars; -applied suspensions for 3 min with a cotton pellet and agitation, allowed to sit for 5 min, then rinsed; -color changes were measured by spectrophotometry. | “the materials used in the study are very promising alternatives to oxidizing bleaching agents”. | 79.2% HAP-whitening effect confirmed. |
| Park et al. 2007 (South Korea) [46] | in vitro | Toothpaste with 15% sodium metaphosphate, toothpaste with 15% nano-HAP. | Sixty sectioned bovine incisors imbedded in resin and mechanically brushed 20,000 strokes (50 strokes/min) with the toothpastes; -a shade guide was used to judge whiteness. | “with the one (dentifrice) with nano-hydroxyapatite, (whitening) is achieved by the adhesion to organic substance on enamel surface”. | 71% HAP-whitening effect confirmed. |
| Kim et al. 2006 (South Korea) [47] | in vitro | Newly developed toothpaste containing nano-sized HAP and two commercial toothpastes (one containing silica and multi-phosphate and the other containing silica and micro-sized HAP). | Sixty-six human extracted human molar crowns imbedded in resin and mechanically brushed 20,000 strokes (50 strokes/min) with the toothpastes; -a shade guide was used to judge whiteness. | “they (new nano-HA toothpaste) had a similar whitening efficacy to commercially available whitening toothpastes”. | 75% HAP-whitening effect confirmed. |
| Park et al. 2006 (South Korea) [48] | in vitro | Four types of toothpaste slurries including 40% calcium carbonate, 15% dicalcium phosphate, 15% sodium metaphosphate, and 12% hydrated silica + 3% nano-HAP. | Eighty natural human teeth imbedded in resin and mechanically brushed 20,000 strokes (50 strokes/min) with the toothpastes; -a shade guide was used to judge whiteness. | “Unlike the result that sodium metaphosphate-included dentifrice has whitened the teeth through the abrasion of the hard tissue, it is judged that the nano-hydroxyapatite have been attached to the hard demineralized tissue and improving the teeth-whitening”. | 75% HAP-whitening effect confirmed. |
| Niwa et al. 2001 (Japan) [49] | in vitro | Toothpastes containing 0%, 3%, and 15% HAP. | Five artificial sintered hydroxyapatite tooth blocks were tested for polishing effects of different HAP concentrations; -thickness loss (nm/cm3/h) was measured with each HAP paste. | “Adding different amounts of hydroxyapatite to toothpaste does not change the polishing properties”. | 66.7% (15% HAP was no different in polishing efficiency than 3% HAP); -statistics were inadequate. |
| Study [Ref.] | Clearly Stated Aims, Objectives | Detailed Explanation of Sample Size Calculation | Detailed Explanation of Sampling Technique | Details of Comparison Group | Detailed Explanation of Methodology | Operator Details | Randomization | Method of Measurement of Outcome | Outcome Assessor Details | Blinding | Statistical Analysis | Presentation of Result | Score | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hojabri 2022 [37] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Shang 2022 [28] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Shang 2021 [39] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Hojabri 2021 [40] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Sarembe 2020 [41] | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 18(100)/24 | 75 |
| Bommer 2018 [42] | 2 | 0 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 17(100)/24 | 71 |
| Jin 2013 [43] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Kim 2011 [44] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Dabanoglu 2009 [45] | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 19 (100)/24 | 79.1 |
| Park 2007 [46] | 2 | 0 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 17 (100)/24 | 71 |
| Kim 2006 [47] | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 18 (100)/24 | 75 |
| Park 2006 [48] | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 18 (100)/24 | 75 |
| Niwa 2001 [49] | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 16 (100)/24 | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limeback, H.; Meyer, F.; Enax, J. Tooth Whitening with Hydroxyapatite: A Systematic Review. Dent. J. 2023, 11, 50. https://doi.org/10.3390/dj11020050
Limeback H, Meyer F, Enax J. Tooth Whitening with Hydroxyapatite: A Systematic Review. Dentistry Journal. 2023; 11(2):50. https://doi.org/10.3390/dj11020050
Chicago/Turabian StyleLimeback, Hardy, Frederic Meyer, and Joachim Enax. 2023. "Tooth Whitening with Hydroxyapatite: A Systematic Review" Dentistry Journal 11, no. 2: 50. https://doi.org/10.3390/dj11020050
APA StyleLimeback, H., Meyer, F., & Enax, J. (2023). Tooth Whitening with Hydroxyapatite: A Systematic Review. Dentistry Journal, 11(2), 50. https://doi.org/10.3390/dj11020050