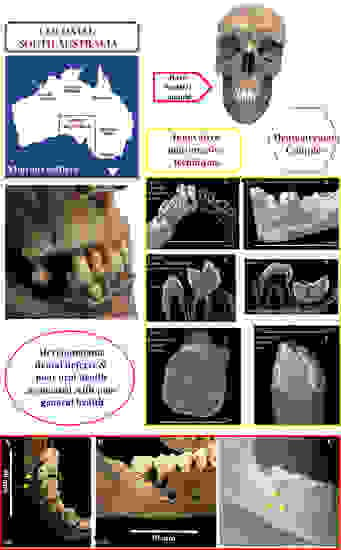
The Oral Health of a Group of 19th Century South Australian Settlers in Relation to Their General Health and Compared with That of Contemporaneous Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials—The Archaeological Sample
2.1.1. The Archaeological Sample—Dentitions
2.1.2. The Archaeological Sample—Ethics
2.2. Methods
2.2.1. Large Volume Micro-CT
2.2.2. Small Volume Micro-CT
2.2.3. Macroscopic Examination
2.2.4. Standard Dental Radiographs
2.2.5. Scoring: Dental and Alveolar Bone Health Categories
| Categories to Be Investigated | Criteria for Identification | Scoring Systems | References |
|---|---|---|---|
| Dental Inventory | Total number of teeth in situ. Antemortem tooth loss: evidence of alveolar tissue healing. Postmortem tooth loss: open socket and no evidence of bone healing | Data were recorded on a visual chart representing the primary/permanent teeth using the FDI (ISO 3950) notation system. (i) tooth type present, (ii) location of healed alveoli, (iii) open socket—location in the alveolar process | [25,26,37,38,39,40] |
| Dental age range | Erupted tooth types present, semi-erupted, and developing teeth in alveolar bones Tooth wear—adult molars only | The London Atlas of tooth eruption and development was used with dental radiographs to identify the stage of eruption and tooth development (0–23.5 years). Adult age range: assessment of the functional age of each molar and the predicted age of the subject based on tooth wear scores set out by Miles (1962). | [5,6,28] |
| Tooth wear | Evidence of enamel loss and/or exposure of dentine on the occlusal surface of the teeth | Category of tooth wear selected from Molnar’s (1971) and Miles’s (1962) criteria charts. | [28,29] |
| Carious lesions (caries—cavity) | (1) Evidence of decay: (a) present on enamel surface only, (b) involving enamel and dentine, (c) decay involving the enamel dentine and the pulp. (2) Identify changes in radiolucency/density of the tooth | Score: (i) tooth type affected (FDI), (ii) location of the carious lesion in relation to the CEJ, (iii) ICDAS/ICCMS category of radiolucency-using dental radiographs and DRRs. Select a category from a visual chart. | [41] |
| Periodontal disease | (i) Evidence of alveolar bone loss (ii) Evidence of morphological changes in the margins of the contours of the alveolar bone of the posterior teeth (buccal surface only) | (i) Measurement taken from the CEJ to the crest of the alveolar bone on the midline of the crown surface (labial/buccal and lingual/palatal). (ii) Alveolar bone status: graded 0–4 using Ogden’s (2008) system via inspection of the margins of the alveolar bone surrounding the posterior teeth. | [42,43,44] |
| Enamel hypoplastic defects (EH) | Evidence of lines or pits in the surfaces of the enamel | Scored using an adaptation of the Enamel Defect Index (EDI): (i) type of EH defect/s, (ii) number of EH defects on the enamel surface, (iii) location of EH defect/s—measurement of the distance of the defect/s in relation to the CEJ. | [45,46] |
| Interglobular dentine (IGD) | Evidence of changes in the density of the dentine structure | Record: the presence of IGD as Yes/No (Micro-CT only) | [47,48] |
2.2.6. Scoring: Evaluation of Intra and Inter-Operator Variations
Intra-Operator Variation
Inter-Operator Variation
2.2.7. Statistical Analysis
2.3. Comparison of Historic Dental Samples from Australian, New Zealand, and British Cemeteries
3. Results
3.1. Reproducibility—Standard Statistical Analysis
3.2. Dental Inventory
Dental Age Range
3.3. Tooth Wear
3.4. Carious Lesions
3.5. Periodontal Disease
3.5.1. Alveolar Bone Loss
3.5.2. Alveolar Bone Status:
3.5.3. Calculus
3.6. Enamel Hypoplasia (EH)
3.7. Interglobular Dentine (IGD)
3.8. Comorbidities and Signs of Skeletal and Dental Changes
3.9. Comparison of Historic Dental Samples from Australian, New Zealand, and British Cemeteries
3.9.1. Demographic Profiles
3.9.2. Dental and Alveolar Bone Health Categories
4. Discussion
4.1. Multiple Methods for Additional Data
4.2. Aim 1—The Oral Health Status of St Mary’s Cemetery Sample
4.2.1. Dental Pathologies
4.2.2. Developmental Dental Defects
4.3. Aim 2: Oral Health Conditions and General Health Status
4.4. Aim 3: St Mary’s Oral Health Findings Compared with Australian, New Zealand, and British Historic Samples
4.5. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Standard Dental Radiographs—Summary of Equipment and Settings Used
References
- Gurr, A.; Kumaratilake, J.; Brook, A.H.; Ioannou, S.; Pate, F.D.; Henneberg, M. Health effects of European colonization: An investigation of skeletal remains from 19th to early 20th century migrant settlers in South Australia. PLoS ONE 2022, 17, e0265878. [Google Scholar] [CrossRef]
- Gurr, A.; Brook, A.H.; Kumaratilake, J.; Anson, T.J.; Pate, F.D.; Henneberg, M. Was it worth migrating to the new British industrial colony of South Australia? Evidence from skeletal pathologies and historic records of a sample of 19th-century settlers. Int. J. Paleopathol. 2022, 37, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.E.; White, F.H. Principles of Anatomy and Oral Anatomy for Dental Students; Churchill Livingstone: Edinburgh, Scotland, 1992. [Google Scholar]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function; Elsevier: Saint Louis, MO, USA, 2012. [Google Scholar]
- AlQahtani, S. The London Atlas: Developing an Atlas of Tooth Development and Testing Its Quality and Performance Measures. Ph.D. Thesis. Queen Mary University of London: London, UK, 2012. [Google Scholar]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Chapman, A.W. History of Dentistry in South Australia 1836–1936; Australian Dental Association, South Australian Branch: Adelaide, SA, Australia, 1937. [Google Scholar]
- Cullinan, M.P.; Ford, P.J.; Seymour, G.J. Periodontal disease and systemic health: Current status. Aust. Dent. J. 2009, 54, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; Lindefjeld Calabi, K.A.; San Martin, L. Systemic Diseases and Oral Health. Dent. Clin. N. Am. 2014, 58, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Rimondini, L. Oral Microbiome, Oral Health and Systemic Health: A Multidirectional Link. Biomedicines 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Beukers, N.G.F.M.; van der Heijden, G.J.M.G.; van Wijk, A.J.; Loos, B.G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60,174 participants in a large dental school in the Netherlands. J. Epidemiol. Community Health 2017, 71, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Desvarieux, M.; Demmer, R.T.; Rundek, T.; Boden-Albala, B.; Jacobs, D.R.; Papapanou, P.N.; Sacco, R.L. Relationship Between Periodontal Disease, Tooth Loss, and Carotid Artery Plaque: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 2003, 34, 2120–2125. [Google Scholar] [CrossRef] [Green Version]
- Elter, J.R.; Champagne, C.M.E.; Offenbacher, S.; Beck, J.D. Relationship of Periodontal Disease and Tooth Loss to Prevalence of Coronary Heart Disease. J. Periodontol. 2004, 75, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. J. Periodontol. 2009, 80, 1021–1032. [Google Scholar] [CrossRef]
- Borgnakke, W.S.; Yl€ostalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84, S135–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, M.; Taylor, G.W.; Awano, S.; Yoshida, A.; Kataoka, S.; Ansai, T.; Nakamura, H. Periodontal disease and pneumonia mortality in haemodialysis patients: A 7-year cohort study. J. Clin. Periodontol. 2018, 45, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Mylotte, J.M. Relationships between Periodontal Disease and Bacterial Pneumonia. J. Periodontol. 1996, 67, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Anson, T.J. The Bioarchaeology of the St. Mary’s Free Ground Burials: Reconstruction of Colonial South Australian Lifeways. Ph.D. Thesis, University of Adelaide, Deparment of Anatomical Sciences, Adelaide, SA, Australia, 2004. [Google Scholar]
- Pate, F.D.; Lucas, T.; Anson, T.J.; Henneberg, M. Bioarchaeology of a mid-late 19th century Anglican community: St Mary’s cemetery, Adelaide, South Australia. Acta Palaeomedica 2021, 2, 97–109. [Google Scholar] [CrossRef]
- Gurr, A.; Higgins, D.; Henneberg, M.; Kumaratilake, J.; O’Donnell, M.B.; McKinnon, M.; Hall, K.A.; Brook, A.H. Investigating the dentoalveolar complex in archaeological human skull specimens: Additional findings with Large Volume Micro-CT compared to standard methods. Int. J. Osteoarchaeol. 2023; early view. [Google Scholar] [CrossRef]
- Nikon Metrology. Products: X-ray and CT Technology: X-ray and CT Systems: 225 kV and 320 kV CT Inspection and Metrology. Available online: https://www.nikonmetrology.com/de/roentgen-und-ct-technik/x-ray-ct-syteme-225kv-und-320kv-ct-inspektion-und-messtechnik (accessed on 20 June 2022).
- Bruker. Position, Scan, Reconstruct and Analyze. Available online: https://www.bruker.com/en/products-and-solutions/microscopes/3d-x-ray-microscopes/skyscan-1272.html (accessed on 22 June 2022).
- Al-Mutairi, R.; Liversidge, H.; Gillam, D.G. Prevalence of Moderate to Severe Periodontitis in an 18-19th Century Sample-St. Bride’s Lower Churchyard (London, UK). Dent. J. 2022, 10, 56. [Google Scholar] [CrossRef]
- FDI World Dental Federation. FDI Two-Digit Notation. Available online: http://www.fdiworldental.org/resources/5_0notation.html (accessed on 22 June 2022).
- Leatherman, G. Two-digit system of designating teeth—FDI submission. Aust. Dent. J. 1971, 16, 394. [Google Scholar] [CrossRef]
- Planmeca. Intraoral X-ray Unit. Available online: https://www.planmeca.com/imaging/intraoral-imaging/intraoral-x-ray-unit/ (accessed on 10 June 2022).
- Miles, A.E.W. Assessment of the Ages of a Population of Anglo-Saxons from Their Dentitions1. Proc. R. Soc. Med. 1962, 55, 881–886. [Google Scholar]
- Molnar, S. Human tooth wear, tooth function and cultural variability. Am. J. Phys. Anthropol. 1971, 34, 175–189. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. 3D Visulaization & Analysis Software: Amiro-Avizo. Available online: https://www.thermofisher.com/au/en/home/industrial/electron-microscopy/electron-microscopy-instruments-workflow-solutions/3d-visualization-analysis-software.html (accessed on 10 June 2022).
- Connell, B.; Rauxloh, P. A Rapid Method for Recording Human Skeletal Data; Museum of London: London, UK, 2003. [Google Scholar]
- Powers, N. Human Osteology Method Statement; Museum of London: London, UK, 2012. [Google Scholar]
- Brothwell, D.R. Digging up Bones: The Excavation, Treatment and Study of the Human Skeletal Remains; British Museum Natural History: London, UK, 1963. [Google Scholar]
- Efremov, J.A. Taphonomy: New Branch of Paleontology. Am. Geol. 1940, 74, 81–93. [Google Scholar]
- Garot, E.; Couture-Veschambre, C.; Manton, D.; Beauval, C.; Rouas, P. Analytical evidence of enamel hypomineralisation on permanent and primary molars amongst past populations. Sci. Rep. 2017, 7, 1710–1712. [Google Scholar] [CrossRef] [Green Version]
- Garot, E.; Couture-Veschambre, C.; Manton, D.; Bekvalac, J.; Rouas, P. Differential diagnoses of enamel hypomineralisation in an archaeological context: A postmedieval skeletal collection reassessment. Int. J. Osteoarchaeol. 2019, 29, 747–759. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Hillson, S. Dental Anthropology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- International Organisation for Standardization; American National Standard; American Dental Association. Specification No. 3950. Designation System for Teeth and Areas of the Oral Cavity. Available online: https://webstore.ansi.org/preview-pages/ADA/preview_ISO+ANSI+ADA+Specification+No.+3950-2010.pdf (accessed on 22 June 2022).
- Kinaston, R.; Willis, A.; Miszkiewicz, J.J.; Tromp, M.; Oxenham, M.F. The Dentition: Development, Disturbances, Disease, Diet, and Chemistry. In Ortner’s Identification of Paleopathological Conditions in Human Skeletal Remains, 3rd ed.; Buikstra, J.E., Ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Pitts, N.B.; Ekstrand, K.R. International Caries Detection and Assessment System (ICDAS) and its International Caries Classification and Management System (ICCMS)—Methods for staging of the caries process and enabling dentists to manage caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Ogden, A.R.; Pinhasi, R.; White, W.J. Gross enamel hypoplasia in molars from subadults in a 16th–18th century London graveyard. Am. J. Phys. Anthropol. 2007, 133, 957–966. [Google Scholar] [CrossRef]
- Perschbacher, S. Periodontal Diseases. In Oral Radiology: Prinicplas and Interpretations; White, S.C., Pharoah, M.J., Eds.; Elsvier: St. Louis, MO, USA, 2014; pp. 299–313. [Google Scholar]
- Riga, A.; Begni, C.; Sala, S.; Erriu, S.; Gori, S.; Moggi-Cecchi, J.; Mori, T.; Dori, I. Is root exposure a good marker of periodontal disease? Bull. Int. Assoc. Paleodont. 2021, 15, 21–30. [Google Scholar]
- Brook, A.H.; Elcock, C.; Hallonsten, A.L.; Poulsen, S.; Andreasen, J.; Koch, G.; Yeung, C.A.; Dosanjh, T. The development of a new index to measure enamel defects. In Dental Morphology; Brook, A.H., Ed.; Academic Press: Sheffield, UK, 2001; pp. 59–66. [Google Scholar]
- Elcock, C.; Lath, D.L.; Luty, J.D.; Gallagher, M.G.; Abdellatif, A.; Bäckman, B.; Brook, A.H. The new Enamel Defects Index: Testing and expansion. Eur. J. Oral Sci. 2006, 114 (Suppl. S1), 35. [Google Scholar] [CrossRef]
- Colombo, A.; Ortenzio, L.; Bertrand, B.; Coqueugniot, H.; KnüSel, C.J.; Kahlon, B.; Brickley, M. Micro-computed tomography of teeth as an alternative way to detect and analyse vitamin D deficiency. J. Archaeol. Sci. Rep. 2019, 23, 390–395. [Google Scholar] [CrossRef]
- Veselka, B.; Brickley, M.B.; D’Ortenzio, L.; Kahlon, B.; Hoogland, M.L.P.; Waters-Rist, A.L. Micro-CT assessment of dental mineralization defects indicative of vitamin D deficiency in two 17th–19th century Dutch communities. Am. J. Phys. Anthropol. 2019, 169, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- StataCorp, L.L.C. Stata, Home: Products: New in Stata. Available online: https://www.stata.com/new-in-stata/ (accessed on 2 December 2022).
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Higginbotham, E.; Associates Pty Ltd. Report on the Excavation of the Cadia Cemetery, Cadia Road, Cadia, NSW, 1997–1998; Higginbotham & Associates Pty Ltd.: Haberfield, NSW, Australia, 2002. [Google Scholar]
- Donlon, D.; Griffin, R.; Casey, M. The Old Sydney Burial Ground: Clues from the dentition about the ancestry, health and diet of the first British settlers of Australia. Australas. Hist. Archaeol. 2017, 35, 43–53. [Google Scholar]
- Buckley, H.R.; Roberts, P.; Kinaston, R.; Petchey, P.; King, C.; Domett, K.; Snoddy, A.M.; Matisoo-Smith, E. Living and dying on the edge of the Empire: A bioarchaeological examination of Otago’s early European settlers. J. R. Soc. N. Z. 2020; ahead-of-print. [Google Scholar] [CrossRef]
- WORD Database. Museum of London. Available online: https://www.museumoflondon.org.uk/collections/other-collection-databases-and-libraries/centre-human-bioarchaeology/osteological-database/post-medieval-cemeteries/post-medieval-cemetery-data (accessed on 23 September 2022).
- Ioannou, S.; Henneberg, M.; Henneberg, R.J.; Anson, T. Diagnosis of Mercurial Teeth in a Possible Case of Congenital Syphilis and Tuberculosis in a 19th Century Child Skeleton. J. Anthropol. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.H. Patterns of molar wear in hunter–gatherers and agriculturalists. Am. J. Phys. Anthropol. 1984, 63, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Littleton, J.; Frohlich, B. Fish-eaters and farmers: Dental pathology in the Arabian Gulf. Am. J. Phys. Anthropol. 1993, 92, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C. Dental wear scoring technique. Am. J. Phys. Anthropol. 1979, 51, 213–217. [Google Scholar] [CrossRef]
- Buikstra, J.E.; Ubelaker, D.H.; Haas, J.; Aftandilian, D.; Field Museum of Natural, H. Standards for Data Collection from Human Skeletal Remains, Proceedings of a Seminar at the Field Museum of Natural History, Organized by Jonathan Haas/volume editors, Jane E. Buikstra and Douglas H. Ubelaker, Assistant Editor, David Aftandilian; Contributions by D. Aftandilian ... [et al.]; Arkansas Archeological Survey: Fayetteville, Arkansas, 1994. [Google Scholar]
- Atar, M.; Körperich, E.J. Systemic disorders and their influence on the development of dental hard tissues: A literature review. J. Dent. 2010, 38, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Seow, K. A study of the development of the permanent dentition of very low birth weight children. Am. Acad. Pediatr. Dent. 1996, 18, 379–384. [Google Scholar]
- Seow, W.K. The effect of medical therapy on dentin formation in vitamin D-resistant rickets. Pediatr. Dent. 1991, 13, 97–102. [Google Scholar]
- Verma, M.; Verma, N.; Sharma, R.; Sharma, A. Dental age estimation methods in adult dentitions: An overview. J. Forensic Dent. Sci. 2019, 11, 57–63. [Google Scholar] [CrossRef]
- The South Australia Register. Dreadful Accident—Coroner’s Inquest; The South Australian Register: Adelaide, SA, Australia, 1859; p. 4. [Google Scholar]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Broers, D.L.M.; Dubois, L.; de Lange, J.; Welie, J.V.M.; Brands, W.G.; Bruers, J.J.M.; de Jongh, A. Financial, psychological, or cultural reasons for extracting healthy or restorable teeth. J. Am. Dent. Assoc. 2022, 153, 761–768.e763. [Google Scholar] [CrossRef]
- Gordon, S.C.; Kaste, L.M.; Barasch, A.; Safford, M.M.; Foong, W.C.; ElGeneidy, A. Prenuptial Dental Extractions in Acadian Women: First Report of a Cultural Tradition. J. Women’s Health 2011, 20, 1813–1818. [Google Scholar] [CrossRef]
- Russell, S.L.; Gordon, S.; Lukacs, J.R.; Kaste, L.M. Sex/Gender differences in tooth loss and edentulism: Historical perspectives, biological factors, and sociologic reasons. Dent. Clin. N. Am. 2013, 57, 317–337. [Google Scholar] [CrossRef]
- Johnson, N.W.; Colman, G. Dental Caries. In A Comprehensive Guide to Clinical Dentistry; Rowe, A.H.R., Ed.; Class Publishing: London, UK, 1989; pp. 112–159. [Google Scholar]
- Drummond, J.C.; Wilbraham, A. The Englishman’s food: A History of Five Centuries of English Diet; Wright, N.C., Ed.; Jonathan Cape: London, UK, 1958. [Google Scholar]
- Holloway, P.J.; Moore, W.J. The role of sugar in the aetiology of dental caries: 1. Sugar and the antiquity of dental caries. J. Dent. 1983, 11, 189–190. [Google Scholar] [CrossRef]
- Mant, M.; Roberts, C. Diet and Dental Caries in Post-Medieval London. Int. J. Hist. Archaeol. 2015, 19, 188–207. [Google Scholar] [CrossRef] [Green Version]
- Miller, I. A Dangerous Revolutionary Force Amongst Us: Conceptualizing Working-Class Tea Drinking in the British Isles, c. 1860–1900. Cult. Soc. Hist. 2013, 10, 419–438. [Google Scholar] [CrossRef]
- Peter, G. Black poison or beneficial beverage?: Tea consumption in colonial Australia. J. Aust. Colonial Hist. 2015, 17, 23–44. [Google Scholar] [CrossRef]
- Wilkinson, J. Adelaide Commercial Report; South Australian Register: Adelaide, SA, Australia, 1853; p. 2. [Google Scholar]
- Winter, G.B. Sugar and Dental Caries. Br. Med. J. 1964, 2, 1595. [Google Scholar] [CrossRef]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, G.; Tayles, N. ‘Abscess cavity’—A misnomer. Int. J. Osteoarchaeol. 1997, 7, 548–554. [Google Scholar] [CrossRef]
- Albandar, J.M.; Streckfus, C.F.; Adesanya, M.R.; Winn, D.M. Cigar, Pipe, and Cigarette Smoking as Risk Factors for Periodontal Disease and Tooth Loss. J. Periodontol. 2000, 71, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.; Schei, O.; Lovdal, A.; Wæehaug, J. Alveolar Bone Loss as a Function of Tobacco Consumption. Acta Odontol. Scand. 1959, 17, 3–10. [Google Scholar] [CrossRef]
- Johnson, G.K. Position Paper: Tobacco Use and the Periodontal Patient. J. Periodontol. 1999, 70, 1419–1427. [Google Scholar] [CrossRef]
- Krall, E.A.; Garvey, A.J.; Garcia, R.I. Alveolar bone loss and tooth loss in male cigar and pipe smokers. J. Am. Dent. Assoc. 1999, 130, 57–64. [Google Scholar] [CrossRef]
- Rivera-Hidalgo, F. Smoking and periodontal disease. Periodontology 2003, 32, 50–58. [Google Scholar] [CrossRef]
- Wilkinson, G.B. South Australia, Its Advantages and Its Resources: Being a Description of That Colony, and a Manual of Information for Emigrants/by George Blakiston Wilkinson; J. Murray: London, UK, 1848. [Google Scholar]
- Ioannou, S.; Henneberg, M. Dental signs attributed to congenital syphilis and its treatments in the Hamann-Todd Skeletal Collection. Anthropol. Rev. 2017, 80, 449–465. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, S.; Sassani, S.; Henneberg, M.; Henneberg, R.J. Diagnosing congenital syphilis using Hutchinson’s method: Differentiating between syphilitic, mercurial, and syphilitic-mercurial dental defects. Am. J. Phys. Anthropol. 2016, 159, 617–629. [Google Scholar] [CrossRef]
- Fearne, J.M.; Bryan, E.M.; Elliman, A.M.; Brook, A.H.; Williams, D.M. Enamel defects in the primary dentition of children born weighing less than 2000 g. Br. Dent. J. 1990, 168, 433–437. [Google Scholar] [CrossRef]
- Nikiforuk, G.; Fraser, D. Etiology of Enamel Hypoplasia and Interglobular Dentin: The Roles of Hypocalcemia and Hypophosphatemia. Metab. Bone Dis. Relat. Res. 1979, 2, 17–23. [Google Scholar] [CrossRef]
- Jump, E.B. Changes within the mandible and teeth in a case of rickets. Am. J. Orthod. Oral Surg. 1939, 25, 484–490. [Google Scholar] [CrossRef]
- Brook, A.H.; Smith, J.M. Hypoplastic enamel defects and environmental stress in a homogeneous Romano-British population. Eur. J. Oral Sci. 2006, 114, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.B.; Brook, A.H. Enamel Hypoplasia and Anomalies of the Enamel. Dent. Clin. N. Am. 1975, 19, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K.; Walls, A.W.G. What dentition assures oral function? Clin. Oral Implant. Res. 2007, 18, 34–45. [Google Scholar] [CrossRef]
- Seraj, Z.; Al-Najjar, D.; Akl, M.; Aladle, N.; Altijani, Y.; Zaki, A.; Al Kawas, S. The Effect of Number of Teeth and Chewing Ability on Cognitive Function of Elderly in UAE: A Pilot Study. Int. J. Dent. 2017, 2017, 5732746–5732748. [Google Scholar] [CrossRef]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. Am. J. Cardiol. 2009, 104, 59–68. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Torres, G.; Levine, M.J. Salivary alpha-amylase: Role in dental plaque and caries formation. Crit. Rev. Oral Biol. Med. 1993, 4, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Farr, J.N.; Khosla, S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2015, 82, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Vilaca, T.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone 2020, 137, 115457. [Google Scholar] [CrossRef]
- Carnevale, V.; Romagnoli, E.; D’Erasmo, E. Skeletal involvement in patients with diabetes mellitus. Diabetes/Metab. Res. Rev. 2004, 20, 196–204. [Google Scholar] [CrossRef]
- Baker, B.J.; Crane-Kramer, G.; Dee, M.W.; Gregoricka, L.A.; Henneberg, M.; Lee, C.; Lukehart, S.A.; Mabey, D.C.; Roberts, C.A.; Stodder, A.L.W.; et al. Advancing the understanding of treponemal disease in the past and present. Am. J. Phys. Anthropol. 2020, 171, 5–41. [Google Scholar] [CrossRef]
- Brickley, M.B.; Ives, R.; Mays, S. The Bioarchaeology of Metabolic Bone Disease; Elsevier Science & Technology: San Diego, CA, USA, 2020. [Google Scholar]
- Buckberry, J.; Crane-Kramer, G. The dark satanic mills: Evaluating patterns of health in England during the industrial revolution. Int. J. Paleopathol. 2022, 39, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Steyn, M.; Van der Merwe, L.; Meyer, A. Infectious diseases and nutritional deficiencies in early industrialized South Africa. Int. J. Paleopathol. 2021, 33, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Nally, C. Cross Bones Graveyard: Excavating the Prostitute in Neo-Victorian Popular Culture. J. Vic. Cult. 2018, 23, 247–261. [Google Scholar] [CrossRef]
- Brook, A.H.; Smith, J.M. The Aetiology of Developmental Defects of Enamel: A Prevalence and Family Study in East London, U.K. Connect. Tissue Res. 1998, 39, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Skelly, E.J. Processes of Industrialisation Influencing the Human Oral Microbiome. Ph.D. Thesis, University of Adelaide, School of Biological Sciences, Adelaide, SA, Australia, 2019. [Google Scholar]
- du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory x-ray micro-computed tomography: A user guideline for biological samples. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wearne, L.S.; Rapagna, S.; Taylor, M.; Perilli, E. Micro-CT scan optimisation for mechanical loading of tibia with titanium tibial tray: A digital volume correlation zero strain error analysis. J. Mech. Behav. Biomed. Mater. 2020, 134, 105336. [Google Scholar] [CrossRef]





| Type of Permanent Tooth | Number of Teeth with Cat. 4 | Number of Teeth with Cat. 5 | Number of Teeth with Cat. 6 | Number of Teeth With Cat. 7 | Number of Teeth With Cat. 8 | Total Number of Each Tooth Type |
|---|---|---|---|---|---|---|
| Cent. Incisor | 13 | 13 | 0 | 0 | 0 | 26 |
| Lat. Incisor | 22 | 18 | 0 | 0 | 0 | 40 |
| Canine | 16 | 14 | 3 | 0 | 0 | 33 |
| P1 | 8 | 6 | 0 | 1 | 0 | 15 |
| P2 | 9 | 1 | 2 | 1 | 0 | 13 |
| M1 | 7 | 1 | 4 | 0 | 0 | 12 |
| M2 | 7 | 3 | 3 | 1 | 0 | 14 |
| M3 | 1 | 1 | 3 | 0 | 1 | 6 |
| Total | 83 | 57 | 15 | 3 | 0 |
| St Mary’s ID | Age Range | Sex | Total Number of Teeth Present | Permanent or Primary Dentition | * Total Number of Teeth Affected by Carious Lesions | Percentage of Teeth Affected by Carious Lesions | Total Number of Carious Lesions Present | ** Antemortem Tooth Loss FDI Notation Number/s of Teeth Lost in Life |
|---|---|---|---|---|---|---|---|---|
| SMB 19 | 6–9 | U | 22 | 7 primary 15 Permanent | 2 | 9% | 3 | None |
| SMB 70 | 6–9 | U | 8 | 2 primary 6 Permanent | 2 | 25% | 3 | None |
| SMB 28 | 10–14 | U | 26 | Permanent | 1 | 4% | 2 | None |
| SMB 79 | 15–18 | U | 25 | Permanent | 8 | 32% | 13 | 36 |
| SMB 05 | 20–30 | F | 6 | Permanent | 4 | 67% | 8 | 11, 12, 13, 14, 15, 16, 18, 21, 22, 23, 24, 25, 26, 27,28, 36, 37, 38, 45, 46, 47, 48 |
| SMB 53C | 30–39 | F | 9 | Permanent | 8 | 89% | 12 | 15, 16, 17, 18, 24, 25, 27, 28, 31, 35, 36, 37, 38, 41, 44, 47, 48 |
| SMB 66B | 30–39 | F | 17 | Permanent | 11 | 65% | 16 | 11 (root only), 13, 16, 18, 26, 28, 36, 37, 38, 46, 47, 48 |
| SMB 73 | 30–39 | M | 19 | Permanent | 17 | 89% | 39 | 15, 17, 18, 24, 26, 27, 36, 37, 38, 46, 47, 48 |
| SMB 06 | 40–49 | M | 24 | Permanent | 12 | 50% | 19 | 28, 34, 35, 36, 38, 48 |
| SMB 09 | 40–49 | M | 22 | Permanent | 1 | 5% | 11 | 11, 27, 38 |
| SMB 57 | 40–49 | M | 25 | Permanent | 8 | 32% | 12 | 25, 28 |
| SMB 61 | 40–49 | F | 7 | Permanent | 4 | 57% | 5 | 11, 12, 15, 16, 17, 18, 21, 22, 24, 26, 27, 28, 334,35, 36, 37,38, 43, 45, 46, 47, 48 |
| SMB 72 | 40–49 | M | 29 | Permanent | 7 | 24% | 15 | None |
| SMB 78 | 40–49 | M | 4 | Permanent | 2 | 50% | 3 | 11, 12, 15, 16, 17, 18, 21, 22, 23, 24, 25, 26, 27, 28, 31, 32, 36, 37, 38, 41, 42, 43, 44, 45, 46, 47, 48 |
| SMB 83 | 40–49 | M | 16 | Permanent | 6 | 38% | 7 | 16, 18, 26, 36, 45, 46 |
| SMB 85 | 40–49 | M | 2 | Permanent | 1 | 50% | 1 | 11, 12, 13, 14, 15, 16, 17, 18, 23, 24, 25, 26, 27, 28, 31, 35, 36, 37, 38, 41, 43, 44, 45, 46, 47, 48 |
| SMB 14 | 50–59 | M | 2 | Permanent | 1 | 50% | 1 | 12, 14, 15, 16, 17, 18, 21, 22, 24, 25, 26, 27, 28, 34, 36, 37, 38, 46, 48 |
| SMB 23 | 50–59 | M | 21 | Permanent | 20 | 95% | 54 | 48 |
| SMB 59 | 50–59 | M | 15 | Permanent | 10 | 67% | 10 | 14, 15, 24, 25, 47 |
| SMB 63 | 50–59 | M | 3 | Permanent | 1 | 33% | 3 | 11, 12, 14, 15, 16, 17, 21, 22, 24, 25, 26, 27, 28, 35, 36, 37, 38, 41, 42, 44, 46, 47, 48 |
| SMB 68 | 50–59 | M | 19 | Permanent | 11 | 58% | 14 | 15, 16, 36, 41, 46 |
| TOTAL | 321 | 137 | 251 | |||||
| Dental Pathology: Calculus | Sample Size (Adults Only) n = | Percentage of Individuals Affected | Number of Teeth Present | Number of Teeth with Calculus Deposits | Percentage of Teeth with Calculus | Mean Number of Teeth Affected |
| 17 | 65% | 240 | 79 | 33% | 7.2 |
| St Mary’s ID | Age range (Skeletal and Eruption Findings) (Years) | Sex | Total Number of Teeth Present | Permanent and/or Primary Dentition | Tooth Type/s Affected by EH Defects FDI Notation | Total Number of Teeth with EH Defects | Percentage of Teeth Affected by EH Defects | Type of EH Defect/s Present |
|---|---|---|---|---|---|---|---|---|
| SMB 58 | 0–2 | U | 11 | All primary teeth | 51, 52, 54, 61, 62, 72, 74, 84 | 8 | 72% | Linear and pits |
| SMB 11 | 0–2 | U | 19 | primary | 53, 63, 73, 83 | 4 | 21% | Pits |
| SMB 04A | 3–5 | U | 19 | primary | 71, 72, 81, 82 | 4 | 21% | Pits |
| SMB 35 | 6–9 | U | 11 | primary | 63 | 1 | 9% | Pits |
| SMB 19 | 6–9 | U | 22 | Mixed 7 primary 15 Permanent | 12, 13, 14, 15, 21, 22, 42, 83 | 8 | 36% | Linear and pit |
| SMB 51 | 10–15 | U | 16 | 1 primary 15 Permanent | 12, 13, 14, 16, 22, 23 24, 25, 26, 34, 36 | 11 | 69% | Linear and pit |
| SMB 52B | 10–15 | U | 17 | 2 primary 15 permanent | 11, 12, 13, 16, 21, 23, 26, 31,32, 33, 36, 14, 42, 43 | 14 | 82% | Linear and pit |
| SMB 70 | 10–15 | U | 8 | 2 primary 6 Permanent | 53, 21, 63, 26 | 4 | 50% | Linear and pits |
| SMB 28 | 10–15 | U | 26 | All Permanent | 11, 12, 15, 16, 22, 23, 25, 26, 27, 31, 32, 33, 34, 35, 41, 42, 43, 44, 45, 47 | 20 | 77% | Linear and pit |
| SMB 79 | 16–19 | U | 25 | Permanent | 13, 23, 27, 33,43 | 5 | 20% | Linear and pit |
| SMB 05 | 20–29 | F | 6 | Permanent | 41, 42 | 2 | 33% | Linear |
| SMB 53C | 30–39 | F | 9 | Permanent | 11, 13, 14, 21, 23 | 5 | 56% | Linear and pit |
| SMB 66B | 30–39 | F | 17 | permanent | 14, 17, 21, 23, 27, 34, 44, 45 | 8 | 47% | Pits |
| SMB 73 | 30–39 | M | 19 | Permanent | 11, 12, 13, 16, 21, 23, 25, 31, 32, 33, 34, 41, 42, 43, 44 | 15 | 79% | Linear and pits |
| SMB 06 | 40–49 | M | 24 | Permanent | 12, 31, 32, 41, 42 | 5 | 21% | Linear |
| SMB 09 | 40–49 | M | 22 | Permanent | 12, 13, 21, 22, 23, 32, 33, 43 | 8 | 36% | Linear and pit |
| SMB 57 | 40–49 | M | 25 | Permanent | 11, 12, 13, 14, 27, 32, 33, 35, 42, 43 | 10 | 40% | Linear and pit |
| SMB 72 | 40–49 | M | 29 | Permanent | 12, 13, 23, 24, 38, 43, 48 | 7 | 24% | Pits |
| SMB 83 | 40–49 | M | 16 | Permanent | 13, 21, 23, 27, 28, 33, 34, 42, 43 | 9 | 56% | Linear and pit |
| SMB 85 | 40–49 | M | 2 | Permanent | 33 | 1 | 50% | Linear and pit 2 |
| SMB 23 | 50–59 | M | 21 | Permanent | 17, 18, 21, 31, 32, 33, 41, 42, 43 | 9 | 43% | Linear and pit |
| SMB 59 | 50–59 | M | 15 | Permanent | 11, 12, 13, 21, 22, 23, 35, 38, 41, 42, 44, 46 | 12 | 80% | Linear and pit |
| SMB 63 | 50–59 | M | 3 | Permanent | 32, 43 | 2 | 67% | Pits |
| SMB 68 | 50–59 | M | 19 | Permanent | 14, 23, 35, 43, 44, 45 | 6 | 42% | Pits |
| Tooth Type | Number of Primary Teeth with EH Defects | Number of Permanent Teeth with EH Defects | Total Number of Each Tooth Type |
|---|---|---|---|
| Cent. Incisor | 4 | 32 | 36 |
| Lat. Incisor | 5 | 29 | 34 |
| Canine | 8 | 44 | 52 |
| P1 | n/a | 18 | 18 |
| P2 | n/a | 12 | 12 |
| M1 | 3 | 10 | 13 |
| M2 | 0 | 8 | 8 |
| M3 | n/a | 5 | 5 |
| Total | 20 | 158 |
| St Mary’s Burial I/D | Sex | Dental Age D = Skeletal Age S = (Years) | Inventory Number of Teeth Present de = Deciduous P = Permanent | Caries Number of Teeth Affected | Periodontal Disease | EH Number of Teeth Affected † Max: | IGD Number of Teeth Affected | Comorbidities Signs of Skeletal and Dental Changes | |
|---|---|---|---|---|---|---|---|---|---|
| Alveolar Bone Status Grade 1–4 Min–Max | Alveolar Bone Loss Number of Teeth Affected | ||||||||
| SMB 58 | U | D= 1–1.5 (±3 months) S= 0–2 | 10 de | 0 | 1 | 0 | 9/10 | 10/10 | (i) Abnormal porosity of the cortical bones of the maxilla and mandible [1]. |
| SMB 04A | U | D= 3.5–4.5 (±6 months) S = 2–4 | 19 de | 0 | 1 | 0 | 4/19 | 0 | (i) Cribra orbitalia Types 3–4 [1]. |
| SMB 19 | U | D = 7.5–8.5 (±1 year) S = 5–9 | 7 de 12 P | 2/19 | 1–2 | 0 | 8/19 | 0 | (i) Cribra orbitalia Types 3–4 [1]. |
| SMB 70 | U | D = 11.5–12.5 (±1 year) S = 8–9 | 2 de 6 P | 6/8 | 1–2 | 0 | 4/8 | 2/2 | (i) Congenital Syphilis, (ii) TB, (iii) mercury toxicity, (iv) abnormal porosity in the cortical bones of the greater wing of sphenoid, maxilla, scapulae, pelvic bones, bilaterally [1,19,56]. |
| SMB 52 B | U | D = 10.5–11.5 (±1 year) S = 8–12 | 2 de 14 P | 0 | 1 | 1/16 | 14/15 | 0 | None seen. |
| SMB 51 | U | D = 10–11. (±1 year) S = 8–12 | 14 de | 0 | 1–3 | 0 | 10/14 | 0 | None seen. |
| SMB 28 | F | D = 15.5–16.5 (±1 year) S = 10–14 | 26 P | 1/26 | 1–4 | 0 | 20/26 | 0 | (i) Cribra orbitalia Type 4, (ii) Possible nutritional deficiency due to abnormal porosity of the cortical bones of the greater wing of the sphenoid and alveolar tissue of the maxilla—bilaterally [1]. |
| SMB 79 | U | D = 15–16 (±1 year) S = 16–18 | 25 P | 13/25 | 1–2 | 0 | 5/25 | 0 | (i) Spina bifida occulta, (ii) evidence of a dental abscess (Figure 3). |
| SMB 05 | F | D = over 23.5 S = 20–29 | 6 P | 4/6 | 1–2 | 0 | 2/5 | 0 | None seen. |
| SMB 53 C | F | D = over 23.5 S = 30–39 | 9 P | 8/9 | 2–3 | 8/9 | 5/9 | 0 | (i) Pitting of the external surface of the Occipital bone, (ii) several vertebral osteophytes- [2]. |
| SMB 66 B | F | D = over 23.5 S = 30–39 | 17 P | 12/17 | 1–4 | 12/17 | 8/17 | 0 | None seen. |
| SMB 73 | M | D = over 23.5 S = 30–39 | 19 P | 17/19 | 1–3 | 15/19 | 18/19 | 7/19 | (i) Torticollis, (iii) spina bifida occulta. |
| SMB 06 | M | D = over 23.5 S = 40–49 | 24 P | 12/24 | 1–4 | 22/24 | 5/24 | 0 | (i) Posterior external surfaces of parietal bones—uneven thickening, possible mild caries sicca. (ii) Vertebral osteophytes and Schmorl’s nodes [1,2,19]. |
| SMB 09 | M | D = over 23.5 S = 40–49 | 21 P | 11/21 | 2–4 | 6/21 | 8/21 | Not micro-CT scanned | (i) Spina bifida occulta. |
| SMB 57 | M | D = over 23.5 S = 40–49 | 25 P | 8/25 | 2–4 | 21/25 | 11/25 | Not micro-CT scanned | (i) Vertebral osteophytes [2]. |
| SMB 61 | F | D = over 23.5 S = 40–49 | 6 P | 4/6 | 2–3 | 3/6 | 0 | Not micro-CT scanned | (i) Spina bifida occulta. |
| SMB 72 | M | D = over 23.5 S = 40–49 | 31 P | 7/31 | 2–3 | 2/31 | 0 | 0 | (i) Evidence of pipe smoker’s tooth wear pattern *. (Figure 2). |
| SMB 78 | M | D = over 23.5 S = 40–49 | 4 P | 2/4 | 0 | 4/4 | 0 | Not micro-CT scanned | (ii) Vertebral osteophytes, (ii) bony growth 20 mm × 10 mm left fibular ossified haemorrhage [2]. |
| SMB 83 | M | D = over 23.5 S = 40–49 | 16 P | 6/16 | 1–3 | 0 | 9/16 | 0 | (i) Spina bifida occulta, (ii) vertebral osteophytes, (iii) bony thickening mid-shaft femur 20 mm × 3 mm, healed trauma antemortem [2]. |
| SMB 85 | M | D = over 23.5 S = 40–49 | 2 P | 1/2 | 3–4 | 2/2 | 1/2 | 0 | (i) Vertebral osteophytes, (ii) eburnation of multiple vertebral facet joints. [2] |
| SMB 14 | M | D = over 23.5 S = 50–59 | 2 P | 1/2 | 2–3 | 2/2 | 0 | Not micro-CT scanned | (i) Multiple vertebral osteophytes, (ii) eburnation of multiple vertebral facet joints, the joints of the ulna, trochlear, and olecranon (bilaterally), femoral head, acetabulum, talus (head), and the navicular [2]. |
| SMB 23 | M | D = over 23.5 S = 50–59 | 21 P | 20/21 | 3–4 | 10/21 | 9/21 | 0 | None seen. |
| SMB 59 | M | D = over 23.5 S = 50–59 | 16 P | 10/16 | 3–4 | 8/16 | 12/16 | Not micro-CT scanned | (i) Evidence of pipe smoker’s tooth wear pattern. (Figure 2). |
| SMB 63 | M | D = over 23.5 S = 50–59 | 3 P | 3/3 | 4 | 3/5 | 2/3 | 1/1 | (i) Spina bifida occulta, (ii) concaved maxillary sinus with signs of new bone growth. |
| SMB 68 | M | D = over 23.5 S = 50–59 | 19 P | 11/19 | 2–4 | 15/19 | 8/19 | 0 | (i) Eburnation of femoral head, acetabulum, talus, and calcaneus [2]. |
| Cemetery: | Total Dental Sample/Total Sample Size | Total Number of Subadults in Dental Sample | Preterm <37 Weeks | Foetal 40 Weeks Post- Partum | Infant 0–11 Months | Subadult 1–5 Years | Subadult 6–11 Years | Adolescent 12–17 Years | Subadult of Unknown Age |
|---|---|---|---|---|---|---|---|---|---|
| St Mary’s Cemetery (SA) 1847–1927 | 40/70 | 22/40 55% | 0 | 0 | 4 | 10 | 6 | 2 | 0 |
| Cadia Cemetery (NSW)1864–1927 | 109/111 | 73/109 67% | 0 | 15 | 25 | 17 | 3 | 4 | 9 |
| Old Sydney Burial Ground (NSW)1792–1820 | 10/10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| St John’s Burial Ground (NZ)1860–1926 | 7/27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cross Bones Burial Ground (UK)1800–1853 | 83/147 | 39/79 47% | 0 | 0 | 8 | 26 | 2 | 1 | 2 |
| Cemetery: | Total Dental Sample/Total Sample Size | Total Number of Adults | Age Group 18–22 Years | Young Adult 23–35 Years | Middle Adult 35–50 Years | Old Adult 50+ Years | Adults of Unknown Age | Adult Male | Adult Female | Adults of Unknown Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| St Mary’s Cemetery (SA)1847–1927 | 40/70 | 18/40 45% | 1 | 3 | 8 | 6 | 0 | 13 | 5 | 0 |
| Cadia Cemetery (NSW)1864–1927 | 109/111 | 36/109 33% | 0 | 7 | 18 | 10 | 1 | 23 | 14 | 0 |
| Old Sydney Burial Ground (NSW)1792–1820 | 10/10 | 10/10 100% | N/A | N/A | N/A | N/A | 10 | 0 | 4 | 6 |
| St John’s Burial Ground (NZ)1860–1926 | 7/27 | 7/7 100% | 0 | 0 | 4 | 4 | 2 | 4 | 3 | 3 |
| Cross Bones Burial Ground (UK)1800–1853 | 83/147 | 44/83 53% | 3 | 4 | 18 | 14 | 5 | 12 | 27 | 5 |
| Cemetery: | Sample Size (Total Number of Individuals) n = | Dental and Alveolar Bone Health Categories Scored | ||||
|---|---|---|---|---|---|---|
| Tooth Wear ‡ Moderate to Heavy | Carious Lesions Present | Periodontal Disease Interdental Alveolar Resorption | Periapical Abscess Present | Enamel Hypoplastic Defects Present | ||
| St Mary’s Cemetery (SA) 1847–1927 | 40 | 14/40 35% | 21/40 53% | 9/40 23% | 1/40 3% | 24/40 60% |
| Cadia Cemetery (NSW) 1864–1927 | 109 | Results not available | 32/109 29% | Results not available | 5/109 5% | Results not available |
| Old Sydney Burial Ground (NSW) 1792–1820 | 10 | 6/10 60% | 4/10 40% | Results not available | Results not available | 7/10 70% |
| St John’s Burial Ground (NZ) 1860–1926 | 7 | 7/7 100% | 6/7 86% | 7/7 100% | 5/7 71% | 6/7 86% |
| Cross Bones Burial Ground (UK) 1800–1853 | 83 | Results not available | 44/83 53% | 42/83 51% | 15/83 18% | 48/83 58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurr, A.; Henneberg, M.; Kumaratilake, J.; Lerche, D.; Richards, L.; Brook, A.H. The Oral Health of a Group of 19th Century South Australian Settlers in Relation to Their General Health and Compared with That of Contemporaneous Samples. Dent. J. 2023, 11, 99. https://doi.org/10.3390/dj11040099
Gurr A, Henneberg M, Kumaratilake J, Lerche D, Richards L, Brook AH. The Oral Health of a Group of 19th Century South Australian Settlers in Relation to Their General Health and Compared with That of Contemporaneous Samples. Dentistry Journal. 2023; 11(4):99. https://doi.org/10.3390/dj11040099
Chicago/Turabian StyleGurr, Angela, Maciej Henneberg, Jaliya Kumaratilake, Derek Lerche, Lindsay Richards, and Alan Henry Brook. 2023. "The Oral Health of a Group of 19th Century South Australian Settlers in Relation to Their General Health and Compared with That of Contemporaneous Samples" Dentistry Journal 11, no. 4: 99. https://doi.org/10.3390/dj11040099
APA StyleGurr, A., Henneberg, M., Kumaratilake, J., Lerche, D., Richards, L., & Brook, A. H. (2023). The Oral Health of a Group of 19th Century South Australian Settlers in Relation to Their General Health and Compared with That of Contemporaneous Samples. Dentistry Journal, 11(4), 99. https://doi.org/10.3390/dj11040099