1. Introduction
The advent of osseointegrated dental implants has revolutionised the treatment of edentulous patients over the past four decades [
1,
2,
3,
4,
5]. High success rates that dental implant therapy achieves in various clinical situations has resulted in implant survival rate of 82.6% up to 15 years [
4]. Despite this, dental implants are still subject to several complications which can compromise this high success rate [
1,
2,
3,
4]. Adler et al. reports that biological complications are the most common complication, responsible for over half (52%) of all encountered complications [
4]. These complications include peri- implant mucositis and peri-implantitis, both of which are inflammatory diseases associated with the placement of dental implants [
1,
2,
3,
4,
6,
7].
In 2017, the ‘World Workshop on Periodontology’ (WWP) defined peri-implant mucositis as “an inflammatory lesion of the soft tissues surrounding an endosseous implant, in the absence of loss of supporting bone or continuing marginal bone loss [
6].” Further, peri-implantitis was defined as “a pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant connective tissue and progressive loss of supportive bone [
7].”
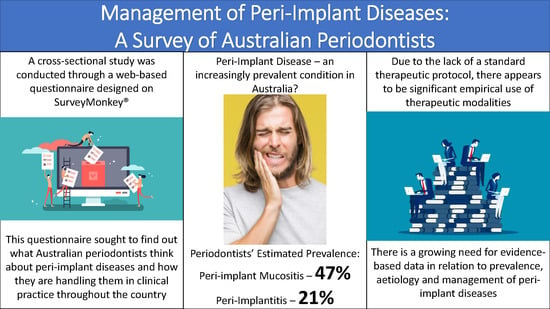
Prevalence of peri-implant mucositis has been reported to be close to 47% whilst peri-implantitis occurred in 20% of implant patients [
8]. Prevention and successful treatment of peri-implant diseases relies on a thorough understanding of the aetiological factors implicated in the pathological condition. A definitive cause-and-effect relationship between bacterial biofilms and peri-implant mucositis has been long established [
6]. This is critical as peri-implant mucositis is considered a precursor for peri- implantitis, although the histopathologic and clinical conditions driving this progression are not yet completely understood [
6,
7]. In addition to bacterial accumulation, several other aetiological factors and risk indicators have been identified in relation to peri-implant diseases, including, poor oral hygiene, history of periodontitis, excess cement, smoking, systemic conditions (i.e., diabetes mellitus), absence of keratinized mucosa, improper design of implant-supported restorations and occlusal overloading [
6,
7,
9].
Despite a growing knowledge base around peri-implant pathologies, the treatment of such conditions remains a clinical challenge. This is primarily due to the lack of evidence-based guidelines available to clinicians in utilising different treatment modalities. Currently, a gold-standard therapeutic protocol for peri-implant pathological lesions does not exist [
10,
11,
12,
13].
Dental implant treatment has risen significantly worldwide over the last few decades, with Australia being described as a “fast expanding market” [
6]. Guo et al. also noted that an increasing proportion of dental implant treatment is being carried out by general dental practitioners, so much so that they predicted that “implant dentistry will become a significant part of contemporary general dental practice in the foreseeable future” [
6]. Prevalence of peri-implant pathologies has also been shown to be positively correlated with the duration for which the implant is in function [
8]. Based on both these factors, it can be projected that biological failures will further affect implant success rates in Australia in the near future.
In 2009, Mattheos et al. surveyed and compared Australian periodontists to British periodontists in relation to their management decisions and attitudes towards peri-implantitis [
2]. Their study concluded that neither country had a universal approach to managing peri-implant pathologies, with significant heterogeneity being reported [
2]. Similar heterogeneity was reported when comparable studies were conducted in Sweden [
14] and the United States of America [
1] (USA).
With an ever-increasing number of implants being used to rehabilitate patients in Australia and those implants being functional units for a greater number of years, it is imperative that a well-defined evidence-based clinical protocol needs to be adopted so as to guide clinicians treating biological complications of implant therapy. In recent years, there has been a concerted effort to increase understanding of peri-implant pathologies, including definitions and diagnostic tools [
3,
6,
7].
This study was conducted to investigate the perceived prevalence along with the aetiological factors for peri-implant pathologies, and to investigate the therapeutic protocols most commonly used to manage these conditions by Australian periodontists.
4. Discussion
Inconsistent definitions, lack of clear clinical parameters and various reporting methods have led to variable reports on the prevalence of peri-implant diseases [
16,
17]. The definitions set out by the WWP in 2017 clarified clinical cut-off points for peri-implant pathologies for both everyday clinical practice and epidemiological studies [
3,
6,
7,
18]. Applying these parameters to the best of their ability, Cosgarea et al. reported the prevalence of peri-implant mucositis to be 46.8% and peri-implantitis to be 18.5 [
19] and 19.83% [
18] for peri-implantitis [
20]. In this study, Australian periodontists reported a disease prevalence of 47% and 21% for peri-implant mucositis and peri-implantitis, respectively. Although these figures closely match those reported previously, [
20] practitioners believed that the disease prevalence in their own patients was significantly lower, at 36% and 14% respectively. Papathanasiou et al. also found that American periodontists felt that the general population had a higher disease prevalence than their own patients. They attributed this finding to possible psychologic factors and response bias [
1]. This discrepancy may also be due to perceived differences in definition of peri-implant disease between epidemiological studies and practitioners in their clinical settings.
Dental Implant therapy is associated with a high success rate and this has been reported by several authors, [
1,
2,
3,
4,
5] including Adler et al. who reported a cumulative implant survival rate of 82.6% over a 9–15-year follow-up period [
4]. Patients in the study conducted by Adler et al. underwent dental therapy in Stockholm, Sweden (1999–2005) [
4]. Participants in this study estimated that approximately 12% of implants needed to be removed due to peri-implantitis in the Australian population. While biological complications are most prevalent, there are other complications which can further affect the implant survival rate. Accounting for other complications, Australian periodontists seem to be experiencing similar cumulative implant survival rates as that reported by Adler et al. [
4].
In the 2017 WWP, Heitz-Mayfield et al. [
6] and Schwarz et al. [
7] elaborated on potential risk factors/indicators with substantial evidence for peri-implant diseases. These included poor plaque control, history of periodontitis, smoking and diabetes. In the current study, Australian periodontists identified bacterial plaque, history of periodontitis and smoking as the three most significant aetiological/predisposing factors behind peri-implant pathology. Several cross-sectional studies have demonstrated that biofilm accumulation is associated with the development of peri-implant mucositis lesions [
21,
22,
23]. Roos-Jansaker et al. studied 218 patients with 999 implants in function over a period of 9- to-14 years, reporting a significant association between plaque scores and peri-implant mucositis [
22]. The progression from peri-implant mucositis to peri-implantitis has been explored in numerous studies [
24,
25]. Costa et al. conducted a retrospective study of 80 patients initially suffering from peri- implant mucositis. Over a five-year period, they found that the incidence of peri-implantitis was lower (18%) in subjects enrolled in a regular maintenance program than those without (43%) [
25]. Roccuzzo et al., [
26] Monje et al. [
27] and Kassebaum et al. [
28] reported that patients who complied to a regular maintenance therapy following implant therapy were significantly less likely to be diagnosed with peri- implantitis, over a period of 10 years, 2 years and 3.8 years, respectively.
Periodontitis is one of the most prevalent diseases in the world, [
28] and it has been found to be associated with peri-implantitis. Karoussis et al. [
29] and Roccuzzo et al. [
26,
30] conducted 10-year longitudinal studies, finding that peri-implantitis incidence in individuals with a history of periodontitis was significantly higher. Roccuzzo et al. also reported that treatment of peri-implantitis was more time-consuming in such patients [
26,
30].
It has been well established that smoking is strongly associated with periodontitis, attachment loss and tooth loss [
7]. It is thought that this effect translates to peri-implant tissues as well. Karoussis et al. found that 18% of all implants in smokers developed peri-implantitis, compared to 6% in nonsmokers, over a period of ten years [
29]. However, several cross-sectional studies have also reported that smokers are not at higher risk for developing peri-implantitis [
31,
32,
33]. These could potentially be due to confounding factors, but nonetheless, Schwarz et al. concluded that there is currently no conclusive evidence that smoking constitutes a risk factor for peri-implantitis [
7].
In 2011, Mombelli et al. conducted a review in which they concluded that the combination of metronidazole and amoxicillin has the potential to overcome a wide range of pathogens often associated with peri-implant disease [
34]. Amoxicillin, [
35] metronidazole [
36] and azithromycin [
37] have all proven to be useful adjuncts in the treatment of peri-implant disease [
12]. Despite their short-term efficacy, there does not appear to be strong evidence that these effects are sustained in the longer term, [
38] and antimicrobial resistance has also been detected in peri-implant biofilms [
39]. The combination of amoxicillin and metronidazole was the most commonly chosen first-choice antibiotic regimen amongst Australian periodontists in this study, particularly amongst those who had been placing implants for more than 20 years or 50+ implants/year.
Practitioners in this study found that oral hygiene instructions, nonsurgical debridement and antimicrobial gel/rinse were effective enough treatment modalities to counter peri-implant mucositis. This notion was supported by Derks et al. who concluded that nonsurgical approaches with thorough mechanical debridement appear to be effective approach for peri-implant mucositis, but outcomes are more guarded in the management of peri-implantitis [
16]. Interestingly, Australian periodontists in our study, were increasingly likely to use surgical approaches and systemic antibiotics to treat peri- implantitis lesions. This may be due to the fact that while nonsurgical treatment is still often performed as part of initial therapy, it has been reported to only be moderately effective in the management of peri-implantitis [
40,
41]. Surgical techniques offer more predictable outcomes, [
42] through improved access and the possibility for resective and regenerative procedures, [
43] with several studies reporting positive outcomes [
44,
45]. Australian periodontists report using surgical techniques often—sometimes to manage peri-implantitis lesions.
Several instruments have been used to debride affected implant surfaces in both surgical and nonsurgical approaches [
46]. Ultrasonic devices and titanium curettes [
11] have been favoured among UK [
2] and American periodontists, [
1] in line with Australian periodontists. Air-abrasive devices are also popular amongst Australian periodontists, although there is little evidence supporting their enhanced cleaning efficacy in the long term, as compared to other instruments [
47,
48]. Surgical regenerative therapy was the least likely treatment protocol used by periodontists treating peri-implant lesions and is rarely utilised by Australian periodontists. Amongst those that use surgical approaches for management of peri-implantitis lesions, the most common regenerative procedure employed was GTR membrane alone, despite some studies demonstrating minimal benefit of barrier membranes when compared to bone-grafting alone [
49,
50].
Supportive maintenance therapy with regular reviews is essential for all patients treated for peri- implant disease [
13] with most Australian periodontists considering a 3-month follow-up as most preferred. A majority of practitioners conducted a radiographic evaluation of the treated site 6- months after treatment. Practitioners with greater experience through years placing implants and implants placed/month were more likely to conduct a radiographic evaluation at 12 months, suggesting that 6 months may be too early to expect radiographic changes in the treated site. Papathanasiou et al. reported similar findings in America, where the majority of practitioners also reported 3-month follow ups and a 6-month radiograph, although they found that less experienced practitioners were more likely to conduct a radiographic evaluation sooner than 6 months [
1].
Although more than three-quarters of practitioners in this study rated their management of peri- implant diseases as moderately effective, it was found that those placing more implants/month were significantly more likely to report their management as mildly effective. The increase in implants placed likely leads to an increase in the number of failures seen, particularly peri-implant diseases, and conditions of varying severity may lead these clinicians to be more cautious in their self- assessment. All but four practitioners reporting that they had updated their training around peri- implant pathology in the last two years and upwards of 90% of practitioners suggested that greater awareness surrounding the management of peri-implant disease is warranted. These figures reflect the continuing uncertainty in this field, with a gold standard management protocol yet to be established, [
10,
11,
12,
13] with one participant aptly stating that “unfortunately, most of the research just tells us what doesn’t work.” While this study has shed light on an increasingly important matter, it does have its limitations, particularly due to its response rate. Although it has a higher response rate than similar studies in the USA [
1] and the UK, [
2] it still falls short of 50% of all eligible periodontists in Australia. While this remains a significant barrier, this study has utilised other means to improve the quality of data, including the use of an electronic format. The electronic format has enabled the collection of accurate and versatile data, with precise prevalence percentages along with a ranking system for aetiology, treatment and instrumentation reported as weighted scores. This electronic format coupled with clear definitions and clinical parameters in accordance with the 2017 WWP have enabled the creation of reliable baseline data for future research in this field.