Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. General Experimental Procedures
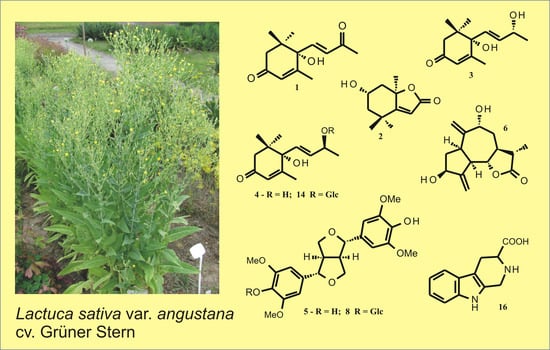
2.3. Plant Material
2.4. Isolation of Chemical Constituents from Leaves of L. sativa L. var. angustana cv. Grüner Stern
2.5. Assessment of the Reducing Capacity of the Plant Material
2.6. DPPH Radical Scavenging Assay
2.7. Sesquiterpene Lactone Analysis
2.8. Quantification of Major Caffeic Acid Derivatives
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobolev, A.P.; Brosio, E.; Gianferri, R.; Segre, A.L. Metabolite profile of lettuce leaves by high-field NMR spectra. Magn. Reson. Chem. 2005, 43, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, L.M.; Contreras, M.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolomic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Viacava, G.E.; Roura, S.I.; Berrueta, L.A.; Iriondo, C.; Gallo, B.; Alonso-Salces, R.M. Characterization of phenolic compounds in green and red oak-leaf lettuce cultivars by UHPLC-DAD-ESI-QtoF/MS using MSE scan mode. J. Mass. Spectrom. 2017, 52, 873–902. [Google Scholar] [CrossRef]
- Viacava, G.E.; Roura, S.I.; López-Márquez, D.M.; Berrueta, L.A.; Gallo, B.; Alonso-Salces, R.M. Polyphenolic profile of butterhead lettuce cultivar by ultrahigh performance liquid chromatography coupled online to UV-visible spectrophotometry and quadrupole time-of-flight mass spectrometry. Food Chem. 2018, 260, 239–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, S.; Liu, B.; Guo, D.; Zheng, B.; Feng, L.; Liu, Y.; Thomás-Barberán, F.A.; Luo, L.; Huang, D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa L.) varieties. Hortic. Res. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, H.; Gillespie, A.L.; Calderwood, D.; Iqbal, H.; Gallagher, C.; Chevallier, O.P.; Elliott, C.T.; Pan, X.; Mirza, B.; Green, B.D. The health promoting bioactivities of Lactuca sativa can be enhanced by genetic modulation of plant secondary metabolites. Metabolites 2019, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Van Treuren, R.; Van Eekelen, H.D.L.M.; Wehrens, R.; De Vos, R.C.H. Metabolite variation in the lettuce gene pool: Towards healthier crop varieties and food. Metabolomics 2018, 14, 146. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.-X.; Zhang, M.-Y.; Han, Y.-Y.; Hao, J.-H.; Liu, C.-J.; Fan, S.-X. Beneficial phytochemicals with anti-tumor potential revealed through metabolic profiling of new red pigmented lettuces (Lactuca sativa L.). Int. J. Mol. Sci. 2018, 19, 1165. [Google Scholar] [CrossRef] [Green Version]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Maas, P.; King, B.M.; Leclercq, E.; Voragen, A.G.J.; De Groot, A. Bitter sesquiterpene lactones from chicory roots. J. Agric. Food Chem. 1990, 38, 1035–1038. [Google Scholar] [CrossRef]
- Mai, F.; Glomb, M.A. Structural and sensory characterization of novel sesquiterpene lactones from iceberg lettuce. J. Agric. Food Chem. 2016, 64, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.B.; Harborne, J.B. The role of sesquiterpene lactones and phenolics in the chemical defence of the chicory plant. Phytochemistry 1985, 24, 2225–2231. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Gumułka, M.; Drożdż, B.; Grabarczyk, H.; Błoszyk, E. Sesquiterpene lactones. XXXVIII. Constituents of Picris echioides L. and their antifeedant activity. Acta Soc. Bot. Pol. 1989, 58, 351–354. [Google Scholar]
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touche, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005, 327, 742–749. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharm. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; van de Putte, B.; Venema, D.P.; Hollman, P.C.H. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef]
- Han, Y.F.; Cao, G.X.; Gao, X.J.; Xia, M. Isolation and characterization of the sesquiterpene lactones from Lactuca sativa L. var. anagustata. Food Chem. 2010, 120, 1083–1088. [Google Scholar] [CrossRef]
- Lissek-Wolf, G.; Lehmann, C.; Huyskens-Keil, S. Die Vielfalt alter Salatsorten—Eine Dokumentation; Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz: Bonn, Germany, 2009; pp. 122–141. (In German) [Google Scholar]
- Kotlińska, T.; Rutkowska-Łoś, A.; Pająkowski, J.; Podyma, W. Informator nt. Starych Odmian Roślin Rolniczych i Ogrodniczych Występujących na Terenie Rzeczpospolitej Polskiej i Możliwościach ich Introdukcji Do Uprawy Jako Odmiany Regionalne i Amatorskie. Ministerstwo Rolnictwa i Rozwoju Wsi; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2015; pp. 27–29. (In Polish)
- Starkenmann, C.; Niclass, I.; Vuichoud, B.; Schweizer, S.; He, X.-F. Occurrence of 2-acetyl-1-pyrroline and its nonvolatile precursors in celtuce (Lactuca sativa L. var. augustana). J. Agric. Food Chem. 2019, 67, 1710–11717. [Google Scholar] [CrossRef]
- Michalska, K.; Michalski, O.; Stojakowska, A. Sesquiterpenoids from roots of Lactuca sativa var. angustana cv. Grüner Stern. Phytochem. Lett. 2017, 20, 425–428. [Google Scholar] [CrossRef]
- De Vries, J.M. Origin and domestication of Lactuca sativa L. Gen. Resour. Crop. Evol. 1997, 44, 165–174. [Google Scholar] [CrossRef]
- Piszczek, P.; Kuszewska, K.; Błaszkowski, J.; Sochacka-Obruśnik, A.; Stojakowska, A.; Zubek, S. Associations between root-inhabiting fungi and 40 species of medicinal plants with potential applications in the pharmaceutical and biotechnological industries. Appl. Soil Ecol. 2019, 137, 69–77. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Beharav, A.; Stojakowska, A.; Ben-David, R.; Malarz, J.; Michalska, K.; Kisiel, W. Variation of sesquiterpene lactone contents in Lactuca georgica natural populations from Armenia. Gen. Resour. Crop. Evol. 2015, 62, 431–441. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Long-Term Cultured Hairy Roots of Chicory—A Rich Source of Hydroxycinnamates and 8-Deoxylactucin Glucoside. Appl. Biochem. Biotechnol. 2013, 171, 1589–1601. [Google Scholar] [CrossRef] [Green Version]
- Kisiel, W.; Michalska, K.; Szneler, E. Norisoprenoids from aerial parts of Cichorium pumilum. Biochem. Syst. Ecol. 2004, 32, 343–346. [Google Scholar] [CrossRef]
- Sung, P.J.; Chen, B.-Y.; Chen, Y.-H.; Chiang, M.Y.; Lin, M.-R. Loliolide: Occurrence of a carotenoid metabolite in the octocoral Briareum excavatum (Briareidae). Biochem. Syst. Ecol. 2010, 38, 116–118. [Google Scholar] [CrossRef]
- Yamano, Y.; Ito, M. Synthesis of Optically Active Vomifoliol and Roseoside Stereoisomers. Chem. Pharm. Bull. 2005, 53, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Çaliş, I.; Kuruüzüm-Uz, A.; Lorenzetto, P.A.; Rüedi, P. (6S)-Hydroxy-3-oxo-α-ionol glucosides from Capparis spinosa fruits. Phytochemistry 2002, 59, 451–457. [Google Scholar] [CrossRef]
- Yajima, A.; Oono, Y.; Nakagawa, R.; Nukada, T.; Yabuta, G. A simple synthesis of four stereoisomers of roseoside and their inhibitory activity on leukotriene release from mice bone marrow-derived cultured mast cells. Bioorg. Med. Chem. 2009, 17, 189–194. [Google Scholar] [CrossRef]
- Xiong, J.; Bui, V.-B.; Liu, X.-H.; Hong, Z.-L.; Yang, G.-X.; Hu, J.-F. Lignans from the stems of Clematis armandii (“Chuan-Mu-Tong”) and their anti-neuroinflammatory activities. J. Ethnopharmacol. 2014, 153, 737–743. [Google Scholar] [CrossRef]
- Shahat, A.A.; Abdel-Azim, N.S.; Pieters, L.; Vlietinck, A.J. Isolation and NMR spectra of syringaresinol-β-D-glucoside from Cressa cretica. Fitoterapia 2004, 75, 771–773. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.Y.; Woo, W.S.; Young, H.S. Isolation of a β-carboline alkaloid from the leaves of Allium tuberosum. Arch. Pharm. Res. 1988, 11, 270–272. [Google Scholar] [CrossRef]
- Ke, R.; Zhu, E.-Y.; Chou, G.-X. A new phenylpropanoid glycoside from Cirsium setosum. Acta Pharm. Sin. 2010, 45, 879–882. [Google Scholar]
- Wang, F.-X.; Deng, A.-J.; Li, M.; Wei, J.-F.; Qin, H.-L.; Wang, A.-P. (3S)-1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid from Cichorium endivia L. induces apoptosis of human colorectal cancer HCT-8 cells. Molecules 2013, 18, 418–429. [Google Scholar] [CrossRef]
- Kisiel, W.; Kohlmünzer, S. Ixerin F from Crepis biennis. Planta Med. 1987, 53, 390. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Miyase, T.; Ueno, A.; Noro, T.; Kuroyanagi, M.; Fukushima, S. Sesquiterpene lactones from Lactuca laciniata. Phytochemistry 1986, 25, 2375–2379. [Google Scholar] [CrossRef]
- Michalska, K.; Szneler, E.; Kisiel, W. Lactuca altaica as a rich source of sesquiterpene lactones. Biochem. Syst. Ecol. 2010, 38, 1246–1249. [Google Scholar] [CrossRef]
- Kisiel, W.; Barszcz, B. Sesquiterpene lactones from Crepis rhoeadifolia. Phytochemistry 1996, 43, 823–825. [Google Scholar] [CrossRef]
- Kisiel, W.; Michalska, K. A new coumarin glucoside ester from Cichorium intybus. Fitoterapia 2002, 73, 544–546. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.-H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, H.Y.; Lee, Y.M.; Kim, Y.H. Anti-inflammatory components of Chrysanthemum indicum flowers. Bioorg. Med. Chem. Lett. 2015, 25, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; Sanz, J.F.; Albiach, R. A sesquiterpene ester from Lactuca serriola. Phytochemistry 1992, 31, 2539–2540. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El Gendy, A.E.-N.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodiv. 2019, 16, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Oliva, R.M.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Allelochemicals from sunflower leaves cv. Peredovick. Phytochemistry 1999, 52, 613–621. [Google Scholar] [CrossRef]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Macias, F.A.; Lacret, R.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Bioactive apocarotenoids from Tectona grandis. Phytochemistry 2008, 69, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lee, C.; Lee, J.W.; Yeon, E.T.; Lee, D.; Han, S.B.; Hong, J.T.; Kim, Y.; Lee, M.K.; Hwang, B.Y. 2-Phenoxychromones and prenylflavonoids from Epimedium koreanum and their inhibitory effects on LPS-induced nitric oxide and interleukin-1β production. J. Nat. Prod. 2014, 77, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shen, L.; Zhang, D.-W.; Dai, S.-J. Two new sesquiterpenoids from Solanum lyratum with cytotoxic activities. Chem. Pharm. Bull. 2009, 57, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Okunade, A.L.; Wiemer, D.F. (-)-Loliolide, an ant-repellent compound from Xanthoxyllum setulosum. J. Nat. Prod. 1985, 48, 472–473. [Google Scholar] [CrossRef]
- Murata, M.; Nakai, Y.; Kawazu, K.; Ishizaka, M.; Kajiwara, H.; Abe, H.; Takeuchi, K.; Ichinose, Y.; Mitsuhara, I.; Mochizuki, A.; et al. Loliolide, a carotenoid metabolite, is a potential endogenous inducer of herbivore resistance. Plant Physiol. 2019, 179, 1822–1833. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.H.; Hwangbo, K.; Zheng, M.S.; Cho, J.H.; Son, J.-K.; Kim, H.Y.; Baek, S.H.; Choi, H.C.; Park, S.Y.; Kim, J.-R. Inhibitory effects of (-)-loliolide on cellular senescence in human dermal fibroblasts. Arch. Pharm. Res. 2015, 38, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, E.; Kim, S.; Kim, D.S.; Kim, J.H.; Chang, S.G.; Choi, J.S.; Park, K.J.; Roh, K.-B.; Lee, J.; et al. Oxidative stress-protective and anti-melanogenic effects of loliolide and ethanol extract from fresh water green algae, Prasiola japonica. Int. J. Mol. Sci. 2018, 19, 2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.-Y.; Liu, C.-H.; Burnouf, T.; Wang, G.-H.; Chang, S.P.; Jassey, A.; Tai, C.-J.; Tai, C.-J.; Huang, C.-J.; Richardson, C.D.; et al. Activity based and fraction guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Anivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Ren, J.; Qin, J.J.; Cheng, X.R.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Five new sesquiterpene lactones from Inula hupehensis. Arch. Pharm. Res. 2013, 36, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-J.; Jin, H.-Z.; Zhu, J.-X.; Fu, J.-J.; Zeng, Q.; Cheng, X.-R.; Zhu, Y.; Shan, L.; Zhang, S.-D.; Pan, Y.-X.; et al. New sesquiterpenes from Inula japonica Thunb. with their inhibitory activities against LPS-induced NO production in RAW264.7 macrophages. Tetrahedron 2010, 66, 9379–9388. [Google Scholar] [CrossRef]
- Dat, N.T.; Jin, X.; Hong, Y.-S.; Lee, J.J. An isoaurone and other constituents from Trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-κB. J. Nat. Prod. 2010, 73, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wei, H.; Jiang, Z.; Li, X.; Jiao, K.; Jia, X.; Hou, Y.; Li, N. Natural potential neuroinflammatory inhibitors from Alhagi sparsifolia Shap. Bioorg. Med. Chem. Lett. 2017, 27, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Gonzalez, S.J.B.; Alejandro, G.J.D.; An, S.S.A. Neuroprotective effect of vomifoliol, isolated from Tarenna obtusifolia Merr. (Rubiaceae), against amyloid-beta1-42-treated neuroblastoma SH-SY5Y cells. 3 Biotech. 2020, 10, 424. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, H.; Saka, M.; Yoshizumi, S.; Yamahara, J.; Matsuda, H. Medicinal foodstuffs. V. Moroheiya. (1): Absolute stereostuctures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae). Chem. Pharm. Bull. 1997, 45, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.Y.; Kim, T.Y.; Hong, G.U.; Kang, H.; Lee, J.-Y.; Park, J.Y.; Kim, S.-C.; Kim, Y.H.; Chung, M.-H.; Kwon, Y.-I.; et al. Inhibitory effects of roseoside and icariside E4 isolated from a natural product mixture (No-ap) on the expression of angiotensin II receptor 1 and oxidative stress in angiotensin II-stimulated H9C2 cells. Molecules 2019, 24, 414. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Kobayashi, E.; Li, S.-H.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Tokuda, H.; Nishino, H.; et al. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002, 50, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Wang, G.-J.; Lee, C.-K.; Kuo, Y.-H.; Chou, C.-H. Inhibitory effects of glycosides from the leaves of Melaleuca quinquenervia on vascular contraction of rats. Planta Med. 2002, 68, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Frankish, N.; de Sousa Menezes, F.; Mills, C.; Sheridan, H. Enhancement of insulin release from the β-cell line INS-1 by an ethanolic extract of Bauhinia variegata and its major constituent roseoside. Planta Med. 2010, 76, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Assefa, A.D.; Choi, S.; Lee, J.E.; Sung, J.-S.; Hur, O.-S.; Ro, N.-Y.; Lee, H.-S.; Jang, S.-W.; Rhee, J.-H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, R.; Yang, Y.; Zhang, M. Isolation, purification and identification of antioxidants in an aqueous aged garlic extract. Food Chem. 2015, 187, 37–43. [Google Scholar] [CrossRef]




| Plant Material | Total Phenolic Content (mg g−1 Dry Weight) GA eq |
|---|---|
| L. sativa cv. Grüner Stern, 8 weeks | 36.36 ± 2.78 |
| 15 weeks | 44.09 ± 3.83 |
| L. sativa cv. Karola, 8 weeks | 41.55 ± 1.55 |
| 15 weeks | 44.46 ± 3.82 |
| L. serriola Münster, 8 weeks | 36.38 ± 5.58 |
| 15 weeks | 46.03 ± 4.47 |
| L. serriola Nantes, 8 weeks | 43.27 ± 1.79 |
| 15 weeks | 46.81 ± 4.21 |
| Plant Material | CTA | 5-CQA | Caffeic Acid | DCTA |
|---|---|---|---|---|
| L. sativa cv. Grüner Stern | 0.121 ± 0.087 a | 0.108 ± 0.023 a | 0.018 ± 0.03 a | 1.023 ± 0.124 a |
| L. sativa cv. Karola | 0.185 ± 0.037 a | 0.246 ± 0.036 b | 0.021 ± 0.007 a | 1.733 ± 0.101 a |
| L. sativa cv. Great Lakes | 0.142 ± 0.01 a | 0.176 ± 0.047 a | 0.038 ± 0.001 a | 1.234 ± 0.026 a |
| L. sativa cv. Amerikanischer Brauner | 0.161 ± 0.02 a | 0.622 ± 0.035 c | 0.047 ± 0.01 a | 2.069 ± 0.075 a |
| L. serriola Münster | 0.560 ± 0.027 b | 0.062 ± 0.004 a | 0.029 ± 0.007 a | 1.888 ± 0.483 a |
| L. serriola Nantes | 0.363 ± 0.055 b | 0.243 ± 0.007 a | 0.027 ± 0.002 a | 2.267 ± 0.537 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malarz, J.; Michalska, K.; Stojakowska, A. Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference? Foods 2021, 10, 59. https://doi.org/10.3390/foods10010059
Malarz J, Michalska K, Stojakowska A. Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference? Foods. 2021; 10(1):59. https://doi.org/10.3390/foods10010059
Chicago/Turabian StyleMalarz, Janusz, Klaudia Michalska, and Anna Stojakowska. 2021. "Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference?" Foods 10, no. 1: 59. https://doi.org/10.3390/foods10010059
APA StyleMalarz, J., Michalska, K., & Stojakowska, A. (2021). Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference? Foods, 10(1), 59. https://doi.org/10.3390/foods10010059