Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals
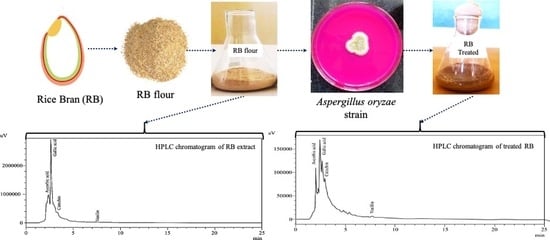
2.2. Isolation of Rice Bran (RB)
2.3. Starter Culture for Solid State Fermentation (SSF)
2.4. SSF of RB
2.5. Evaluation of Phytochemical Composition
2.5.1. Total Phenolic Content (TPC)
2.5.2. Determination of Saponins
2.5.3. Determination of Steroids
2.5.4. Determination of Flavonoid
2.5.5. Condensed Tannin Content (CTC)
2.5.6. Determination of Coumarins
2.5.7. Alkaloids
- Wagner’s test: URB and FRB extracts (2 mL) were treated with Wagner’s reagent (2 mL), the formation of precipitate (reddish-brown) confirmed alkaloids in sample extract.
- Mayer’s test: To URB and FRB extract (1 mL), Mayer’s reagent (2 mL) was added, and precipitate (dull white) confirmed alkaloids presence in sample extract.
- Hager’s test: To URB and FRB extract (1 mL), Hager’s reagent (3 mL) was added, and the formation of precipitate (yellow) confirmed alkaloids in sample extract.
2.5.8. Qualitative and Quantitative High Performance Liquid Chromatography (HPLC) Analysis
2.6. Assessment of Antioxidant Properties in URB and FRB
2.6.1. DPPH (2,2-Diphenyl–1′ picrylhydrazyl) Assay
2.6.2. ABTS Assay
2.6.3. HFRSA Assay
2.6.4. Total Antioxidant Capacity (TAC)
2.6.5. Reducing Power Assay (RPA)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of SSF on Phytochemicals and TPC
3.2. Effect of SSF on CTC
3.3. Effect of SSF on Specific Bioactive Constituents
3.4. Effect of SSF on Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agricultural Organization of United Nations). 2017. Available online: http://faostat.fao.org/beta/en/#data/QC (accessed on 20 May 2019).
- Pourali, O.; Asghari, F.S.; Yoshida, H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem. Eng. J. 2010, 160, 259–266. [Google Scholar] [CrossRef]
- Parrado, J.; Miramontes, E.; Jover, M.; Gutierrez, J.F.; de Teran, L.C.; Bautista, J. Preparation of a rice bran enzymatic extract with potential use as functional food. Food Chem. 2006, 98, 742–748. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I.; Anwar, F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005, 93, 265–272. [Google Scholar] [CrossRef]
- Anal, A. (Ed.) Food Processing By-Products and Their Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2018; Incorporated. [Google Scholar]
- Tosuner, Z.V.; Taylan, G.G.; Özmıhçı, S. Effects of rice husk particle size on biohydrogen production under solid state fermentation. Int. J. Hydrog. Energy. 2019, 44, 18785–18791. [Google Scholar] [CrossRef]
- Postemsky, P.D.; Bidegain, M.A.; Lluberas, G.; Lopretti, M.I.; Bonifacino, S.; Landache, M.I.; Omarini, A.B. Biorefining via solid-state fermentation of rice and sunflower by-products employing novel monosporic strains from Pleurotus sapidus. Bioresour Technol. 2019, 289, 121692. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Punia, S. Enhancement of bioactive compounds in barley cultivars by solid substrate fermentation. Food Meas. 2017, 11, 1355–1361. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Bhatti, M.S. Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour. Effic. Technol. 2016, 2, 148–157. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Fermented pearl millet (Pennisetum glaucum) with in vitro DNA damage protection activity, bioactive compounds and antioxidant potential. Food Res. Int. 2017, 100, 204–210. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S.; Kaur, M. Effect of duration of solid state fermentation by Aspergillus awamorinakazawa on antioxidant properties of wheat cultivars. LWT Food Sci. Technol. 2016, 71, 323–328. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Long, K. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal. Agric. Biotechnol. 2015, 4, 33–38. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Yasui, M.; Oda, K.; Masuo, S.; Hosoda, S.; Katayama, T.; Maruyama, J.; Takaya, N.; Takeshita, N. Invasive growth of Aspergillus oryzae in rice koji and increase of nuclear number. Fungal Biol. Biotechnol. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomic Profiles of Aspergillus oryzae and Bacillus amyloliquefaciens During Rice Koji Fermentation. Molecules 2016, 21, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, K.; Kanda, Y.; Ikeda, S.S.; Sakaki, R.; Nonomura, T.; Kobayashi, M.; Osaki, M.; Shikanai, M.; Kobayashi, H.; Okada, F. Fermented Brown Rice and Rice Bran with Aspergillus oryzae (FBRA) Prevents Inflammation-Related Carcinogenesis in Mice, through Inhibition of Inflammatory Cell Infiltration. Nutrients 2015, 7, 10237–10250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trease, G.E.; Evans, E.C. Pharmacognosy, 13th ed.; Bailliere Tindall: London, UK, 1996; pp. 282–396. [Google Scholar]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Comparison of wheat flours grown at different locations for their antioxidant properties. Food Chem. 2004, 86, 11–16. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Bioactive profile, free-radical scavenging potential, DNA damage protection activity, and mycochemicals in Aspergillus awamori (MTCC 548) extracts: A novel report on filamentous fungi. 3 Biotech 2017, 7, 164. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. Effect of processing methods and storage time on the content of bioactive compounds in blue honeysuckle berry purees. Agronomy 2019, 9, 860. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The effect of the addition of blue honeysuckle berry juice to apple juice on the selected quality characteristics, anthocyanin stability, and antioxidant properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004, 69, R146–R149. [Google Scholar] [CrossRef]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins–a current perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.G.; Furlong, E.B. Effect of particle size and ammonium sulfate concentration on rice bran fermentation with the fungus Rhizopus oryzae. Bioresour. Technol. 2012, 123, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Kaur, M.; Sogi, D.S.; Purewal, S.S. A comparative study of phytochemicals, antioxidant potential and in-vitro DNA damage protection activity of different oat (Avena sativa) cultivars from India. Food Measure. 2019, 13, 347–356. [Google Scholar] [CrossRef]
- Kaur, P.; Dhull, S.B.; Sandhu, K.S.; Salar, R.K.; Purewal, S.S. Tulsi (Ocimumtenuiflorum) seeds: In vitro DNA damage protection, bioactive compounds and antioxidant potential. Food Measure. 2018, 12, 1530–1538. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Lee, I.H.; Hung, Y.H.; Chou, C.C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008, 121, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Belefant-Miller, H.; Grace, S.C. Variations in bran carotenoid levels within and between rice subgroups. Plant Foods Hum. Nutr. 2010, 65, 358–363. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov. Food Sci. Emerg. Technol. 2007, 8, 197–204. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Liang, C.H.; Syu, J.L.; Mau, J.L. Antioxidant properties of solid-state fermented adlay and rice by Phellinus linteus. Food Chem. 2009, 116, 841–845. [Google Scholar] [CrossRef]
- Ng, C.C.; Wang, C.Y.; Wang, Y.P.; Tzeng, W.S.; Shyu, Y.T. Lactic acid bacterial fermentation on the production of functional antioxidant herbal AnoectochilusformosanusHayata. J. Biosci. Bioeng. 2011, 111, 289–293. [Google Scholar] [CrossRef] [PubMed]


| Phytochemical | URB | FRB (4th Day) |
|---|---|---|
| Coumarins | + | + |
| Flavonoids | - | - |
| Saponin | - | - |
| Steroid | - | - |
| Alkaloids | - | - |
| Fermentation Time (Days) | TPC (g GAE/g dwb) | Percent (%) Change in TPC after SSF | CTC (mg CE/g dwb) | Percent (%) Change in CTC after SSF |
|---|---|---|---|---|
| URB | 1.08 ± 0.13 a | -- | 34.6 ± 0.06 a | -- |
| 1 | 4.15 ± 0.09 b | ↑284% | 261 ± 0.05 d | ↑652% |
| 2 | 5.11 ± 0.10 c | ↑373% | 227 ± 0.07 c | ↑555% |
| 3 | 6.52 ± 0.11 e | ↑503% | 365 ± 0.13 g | ↑952% |
| 4 | 8.83 ± 0.21 g | ↑717% | 295 ± 0.11 f | ↑750% |
| 5 | 6.96 ± 0.34 f | ↑544% | 269 ± 0.02 e | ↑675% |
| 6 | 6.37 ± 0.19 d,e | ↑489% | 268 ± 0.07 e | ↑674% |
| 7 | 5.86 ± 0.08 d | ↑442% | 159 ± 0.09 b | ↑358% |
| Compounds | URB | FRB (4th Day) |
|---|---|---|
| Ascorbic acid (μg/g) | 11.1 a | 12.7 b |
| Gallic acid (μg/g) | 14.8 a | 23.3 b |
| Catechin (μg/g) | 9.6 b | 2.8 b |
| Vanillin (μg/g) | 5.8 a | 1.2 a |
| Fermentation Time (Days) | DPPH (% Inhibition) | ABTS (% Inhibition) | TAC (mg AAE/g dwb) | HFRSA (% Inhibition) | RPA (mg QE/g dwb) |
|---|---|---|---|---|---|
| URB | 75.4 ± 0.11 a | 35.3 ± 0.48 a | 7.3 ± 0.46 a | 13.3 ± 0.90 a | 0.7 ± 0.18 a |
| 1 | 77.8 ± 0.33↑3.08 e | 75.8 ± 0.89↑116.5 d | 9.7 ± 0.32↑31 b | 28.8 ± 0.42↑116 e | 2.7 ± 0.39↑260 b |
| 2 | 78.5 ± 0.24↑4.12 d,e | 78.5 ± 0.77↑123.5 e | 13.5 ± 0.38↑87 c | 25.3 ± 0.66↑90 c | 3.5 ± 0.16↑368 c |
| 3 | 83.1 ± 0.20↑10.12 f | 79.8 ± 0.54↑127.9 f | 14.7 ± 0.19↑99 d,e | 25.9 ± 0.48↑94 c | 8.5 ± 0.22↑1040 e |
| 4 | 85.4 ± 0.23↑12.66 g | 82.7 ± 0.71↑136.5 g | 15.4 ± 0.24↑103 f | 28.5 ± 0.24↑114 e | 16.5 ± 0.24↑2102 g |
| 5 | 77 ± 0.19↑2.09 d | 75.2 ± 0.85↑114.6 d | 14.8 ± 0.61↑101 e | 26.1 ± 0.56↑96 d | 12.6 ± 0.21↑1589 f |
| 6 | 76.2 ± 0.56↑1.046 c | 70.7 ± 0.42↑101.9 c | 14.5 ± 0.53↑97 d | 24.7 ± 0.53↑85 c | 8.8 ± 0.27↑1082 e |
| 7 | 75.5 ± 0.17↑0.066 b | 69.5 ± 0.23↑98.6 b | 13.9 ± 0.39↑88 c | 20.2 ± 0.85↑51 b | 6.9 ± 0.34↑826 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods 2021, 10, 70. https://doi.org/10.3390/foods10010070
Punia S, Sandhu KS, Grasso S, Purewal SS, Kaur M, Siroha AK, Kumar K, Kumar V, Kumar M. Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods. 2021; 10(1):70. https://doi.org/10.3390/foods10010070
Chicago/Turabian StylePunia, Sneh, Kawaljit Singh Sandhu, Simona Grasso, Sukhvinder Singh Purewal, Maninder Kaur, Anil Kumar Siroha, Krishan Kumar, Vikas Kumar, and Manoj Kumar. 2021. "Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential" Foods 10, no. 1: 70. https://doi.org/10.3390/foods10010070
APA StylePunia, S., Sandhu, K. S., Grasso, S., Purewal, S. S., Kaur, M., Siroha, A. K., Kumar, K., Kumar, V., & Kumar, M. (2021). Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods, 10(1), 70. https://doi.org/10.3390/foods10010070