Inactivation of Foodborne Viruses by UV Light: A Review
Abstract
:1. Introduction
2. Definition of Viruses
3. The Most Relevant Foodborne Viruses
3.1. Human Noroviruses
3.2. Hepatitis A Virus
3.3. Hepatitis E Virus
3.4. Laboratory Surrogates Used to Study Foodborne Viruses
3.5. Other Important Foodborne Viruses
4. Mechanism of Viral Inactivation by UV, and Genome Repair Mechanisms
4.1. Generalities Regarding Inactivation by UV
4.2. Nucleic Acid
4.3. Protein Damage
4.4. Host Repair Mechanisms
5. Impact of UV Treatment on Foods and Food-Related Matrices (Liquids and Surfaces)
5.1. Non-Food Liquids
5.2. Liquid Foods
5.3. Meat Products
5.4. Fruits and Vegetables
5.5. Other Food Types
5.6. Food Contact Surfaces
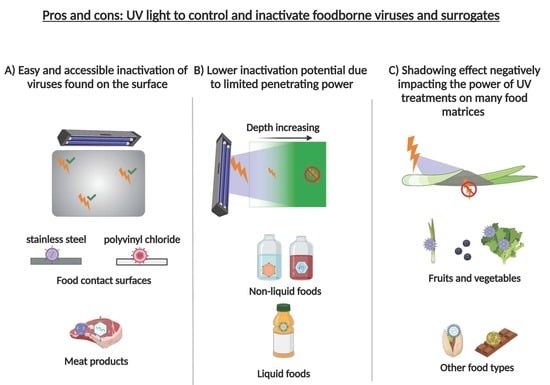
6. Limitations of UV Treatment
7. Legislation
8. Conclusions and Future Trends
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schalk, S.; Adam, V.; Arnold, E.; Brieden, K.; Voronov, A.; Witzke, H.D. UV-lamps for disinfection and advanced oxidation-lamp types, technologies and applications. IUVA 2006, 8, 32–37. [Google Scholar]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Decontamination Methods to Prolong the Shelf-life of Minimally Processed Vegetables, State-of-the-art. Crit. Rev. Food Sci. Nutr. 2008, 48, 487–495. [Google Scholar] [CrossRef]
- McDonald, K.; Curry, R.; Clevenger, T.; Unklesbay, K.; Eisenstark, A.; Golden, J.; Morgan, R. A comparison of pulsed and continuous ultraviolet light sources for the decontamination of surfaces. IEEE Trans. Plasma Sci. 2000, 28, 1581–1587. [Google Scholar] [CrossRef]
- Bohrerova, Z.; Shemer, H.; Lantis, R.; Impellitteri, C.A.; Linden, K.G. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Res. 2008, 42, 2975–2982. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry. Pure Appl. Chem. 1996, 68, 2223–2286. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Bolton, J.R. An Approach to Standardize Methods for Fluence Determination in Bench-Scale Pulsed Light Experiments. Food Bioprocess Technol. 2016, 9, 1040–1048. [Google Scholar] [CrossRef]
- Bolton, J.R. Ultraviolet Applications Handbook, 3rd ed.; ICC Lifelong Learn Inc.: Edmonton, AB, Canada, 2013. [Google Scholar]
- Water-Technology. Catskill-Delaware UV Water Treatment Facility. Available online: https://www.water-technology.net/projects/catskill-delaware-ultraviolet-water-treatment-facility/ (accessed on 4 October 2021).
- Fan, X.; Huang, R.; Chen, H. Application of ultraviolet C technology for surface decontamination of fresh produce. Trends Food Sci. Technol. 2017, 70, 9–19. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Available online: https://apps.who.int/iris/handle/10665/199350 (accessed on 1 November 2021).
- Van Boxstael, S.; Habib, I.; Jacxsens, L.; De Vocht, M.; Baert, L.; Van De Perre, E.; Rajkovic, A.; Galvez, F.L.; Sampers, I.; Spanoghe, P.; et al. Food safety issues in fresh produce: Bacterial pathogens, viruses and pesticide residues indicated as major concerns by stakeholders in the fresh produce chain. Food Control. 2013, 32, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Canadian Food Inspection Agency. Food Recall Warnings and Allergy Alerts. Available online: https://inspection.canada.ca/food-recall-warnings-and-allergy-alerts/eng/1351519587174/1351519588221 (accessed on 1 November 2021).
- U.S. Food & Drug Administration. Recalls, Market Withdrawals, & Safety Alerts. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts (accessed on 1 November 2021).
- U.S. Food & Drug Administration. Archive for Recalls, Market Withdrawals & Safety Alerts. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/archive-recalls-market-withdrawals-safety-alerts (accessed on 1 November 2021).
- World Health Organization & Food and Agriculture Organization of the United Nations. Viruses in Food: Scientific Advice to Support Risk Management Activities: Meeting Report. Available online: https://apps.who.int/iris/handle/10665/44030 (accessed on 1 November 2021).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Burden of Foodborne Diseases in the WHO European Region. 2017. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/food-safety/publications/2017/the-burden-of-foodborne-diseases-in-the-who-european-region-2017 (accessed on 1 November 2021).
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Bouwknegt, M.; Rutjes, S.; Willems, K.A.; Moloney, R.; Husman, A.M.D.R.; Kaupke, A.; Legaki, E.; et al. Harmonised Investigation of the Occurrence of Human Enteric Viruses in the Leafy Green Vegetable Supply Chain in Three European Countries. Food Environ. Virol. 2012, 4, 179–191. [Google Scholar] [CrossRef]
- Lopman, B.; Gastañaduy, P.; Park, G.W.; Hall, A.J.; Parashar, U.D.; Vinje, J. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2012, 2, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Verhaelen, K.; Bouwknegt, M.; Carratalà, A.; Lodder-Verschoor, F.; Diez-Valcarce, M.; Rodríguez-Lázaro, D.; Husman, A.M.D.R.; Rutjes, S.A. Virus transfer proportions between gloved fingertips, soft berries, and lettuce, and associated health risks. Int. J. Food Microbiol. 2013, 166, 419–425. [Google Scholar] [CrossRef]
- Chatziprodromidou, I.P.; Bellou, M.; Vantarakis, G.; Vantarakis, A. Viral outbreaks linked to fresh produce consumption: A systematic review. J. Appl. Microbiol. 2018, 124, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Nasheri, N.; Vester, A.; Petronella, N. Foodborne viral outbreaks associated with frozen produce. Epidemiol. Infect. 2019, 147, e291. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.; Faber, M.; Wilking, H.; Haller, S.; Höhle, M.; Schielke, A.; Ducomble, T.; Siffczyk, C.; Merbecks, S.S.; Fricke, G.; et al. Large multistate outbreak of norovirus gastroenteritis associated with frozen strawberries, Germany, 2012. Eurosurveillance 2014, 19, 20719. [Google Scholar] [CrossRef] [Green Version]
- Scavia, G.; Alfonsi, V.; Taffon, S.; Escher, M.; Bruni, R.; De Medici, D.; Di Pasquale, S.; Guizzardi, S.; Cappelletti, B.; Iannazzo, S.; et al. A large prolonged outbreak of hepatitis A associated with consumption of frozen berries, Italy, 2013–14. J. Med. Microbiol. 2017, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Severi, E.; Verhoef, L.; Thornton, L.; Guzman-Herrador, B.R.; Faber, M.; Sundqvist, L.; Rimhanen-Finne, R.; Roque-Afonso, A.M.; Ngui, S.L.; Allerberger, F.; et al. Large and prolonged food-borne multistate hepatitis A outbreak in Europe associated with consumption of frozen berries, 2013 to 2014. Eurosurveillance 2015, 20, 21192. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, C.; Vogt, T.M.; Armstrong, G.L.; Vaughan, G.; Weltman, A.; Nainan, O.V.; Dato, V.; Xia, G.; Waller, K.; Amon, J.; et al. An Outbreak of Hepatitis A Associated with Green Onions. New Engl. J. Med. 2005, 353, 890–897. [Google Scholar] [CrossRef]
- Meghnath, K.; Team, O.; Hasselback, P.; McCormick, R.; Prystajecky, N.; Taylor, M.; McIntyre, L.; Man, S.; Whitfield, Y.; Warshawsky, B.; et al. Outbreaks of Norovirus and Acute Gastroenteritis Associated with British Columbia Oysters, 2016–2017. Food Environ. Virol. 2019, 11, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.W.; Calci, K.R.; Marchant-Tambone, J.G.; Burkhardt, W. Detection and molecular characterization of norovirus from oysters implicated in outbreaks in the US. Food Microbiol. 2016, 59, 76–84. [Google Scholar] [CrossRef]
- Wall, R.; Dymond, N.; Bell, A.; Thornley, C.; Buik, H.; Cumming, D.; Petersen, N. Two New Zealand outbreaks of norovirus gastroenteritis linked to commercially farmed oysters. N. Z. Med. J. 2011, 124, 63–71. [Google Scholar] [PubMed]
- Pontrelli, G.; Boccia, D.; DI Renzi, M.; Massari, M.; Giugliano, F.; Celentano, L.P.; Taffon, S.; Genovese, D.; DI Pasquale, S.; Scalise, F.; et al. Epidemiological and virological characterization of a large community-wide outbreak of hepatitis A in southern Italy. Epidemiol. Infect. 2008, 136, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Viray, M.A.; Hofmeister, M.G.; Johnston, D.I.; Krishnasamy, V.P.; Nichols, C.; Foster, M.A.; Balajadia, R.; Wise, M.E.; Manuzak, A.; Lin, Y.; et al. Public health investigation and response to a hepatitis A outbreak from imported scallops consumed raw—Hawaii, 2016. Epidemiol. Infect. 2018, 147, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurav, Y.K.; Babu, G.R.; Vinu, K.P.; Lole, K.S. Suspected spread of hepatitis A virus from a restaurant among adults in rural area of the Kerala state, India. Epidemiol. Infect. 2019, 147, e210. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; De Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, e04886. [Google Scholar] [CrossRef]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Proroga, Y.T.R.; De Medici, D.; Capuano, F.; Iaconelli, M.; della Libera, S.; Suffredini, E. First Detection of Hepatitis E Virus in Shellfish and in Seawater from Production Areas in Southern Italy. Food Environ. Virol. 2018, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Miyamura, T.; Takeda, N. Detection of hepatitis E virus RNA from the bivalve Yamato-Shijimi (Corbicula japonica) in Japan. Am. J. Trop. Med. Hyg. 2007, 76, 170–172. [Google Scholar] [CrossRef] [Green Version]
- Purpari, G.; Macaluso, G.; Di Bella, S.; Gucciardi, F.; Mira, F.; Di Marco, P.; Lastra, A.; Petersen, E.; La Rosa, G.; Guercio, A. Molecular characterization of human enteric viruses in food, water samples, and surface swabs in Sicily. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2019, 80, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brassard, J.; Gagné, M.-J.; Généreux, M.; Côté, C. Detection of Human Food-Borne and Zoonotic Viruses on Irrigated, Field-Grown Strawberries. Appl. Environ. Microbiol. 2012, 78, 3763–3766. [Google Scholar] [CrossRef] [Green Version]
- Maunula, L.; Kaupke, A.; Vasickova, P.; Söderberg, K.; Kozyra, I.; Lazic, S.; van der Poel, W.H.; Bouwknegt, M.; Rutjes, S.; Willems, K.A.; et al. Tracing enteric viruses in the European berry fruit supply chain. Int. J. Food Microbiol. 2013, 167, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yiğin, A.; Ünlü, O.; Altun, S.K. Farklı Hayvanlardan Elde Edilen Çiğ Sütlerde HEV RNA Miktarının ve Genotiplerinin Tespiti. Mikrobiyoloji Bulteni 2019, 53, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dziedzinska, R.; Krzyzankova, M.; Bena, M.; Vasickova, P. Evidence of Hepatitis E Virus in Goat and Sheep Milk. Viruses 2020, 12, 1429. [Google Scholar] [CrossRef] [PubMed]
- Montone, A.M.I.; De Sabato, L.; Suffredini, E.; Alise, M.; Zaccherini, A.; Volzone, P.; Di Maro, O.; Neola, B.; Capuano, F.; Di Bartolo, I. Occurrence of HEV-RNA in Italian Regional Pork and Wild Boar Food Products. Food Environ. Virol. 2019, 11, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.G.; Soares, V.M.; Gil De Souza, F.; Tadielo, L.E.; dos Santos, E.A.R.; Brum, M.C.S.; Henzel, A.; Duval, E.H.; Spilki, F.R.; Da Silva, W.P. Hepatitis A Virus, Hepatitis E Virus, and Rotavirus in Foods of Animal Origin Traded at the Borders of Brazil, Argentina, and Uruguay. Food Environ. Virol. 2018, 10, 365–372. [Google Scholar] [CrossRef]
- Malek, M.; Barzilay, E.; Kramer, A.; Camp, B.; Jaykus, L.; Escudero-Abarca, B.; Derrick, G.; White, P.; Gerba, C.; Higgins, C.; et al. Outbreak of Norovirus Infection among River Rafters Associated with Packaged Delicatessen Meat, Grand Canyon, 2005. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 31–37. [Google Scholar] [CrossRef]
- Prasad, B.V.V.; Schmid, M.F. Principles of Virus Structural Organization. Adv. Exp. Med. Biol. 2012, 726, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Viruses, I.C.o.T.o. Introduction to Virus Taxonomy. Available online: https://talk.ictvonline.org/taxonomy/w/ictv-taxonomy (accessed on 14 September 2021).
- Mateu, M.G. Introduction: The Structural Basis of Virus Function. Subcell Biochem. 2013, 68, 3–51. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Liu, C.; Yount, B.; Gully, K.; Yang, Y.; Auerbach, A.; Peng, G.; Baric, R.; Li, F. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020, 16, e1008392. [Google Scholar] [CrossRef] [PubMed]
- Haralampiev, I.; Prisner, S.; Nitzan, M.; Schade, M.; Jolmes, F.; Schreiber, M.; Loidolt-Krüger, M.; Jongen, K.; Chamiolo, J.; Nilson, N.; et al. Selective flexible packaging pathways of the segmented genome of influenza A virus. Nat. Commun. 2020, 11, 4355. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Schlub, T.; Holmes, E.C. An Allometric Relationship between the Genome Length and Virion Volume of Viruses. J. Virol. 2014, 88, 6403–6410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, K.F.; Steward, G.F.; Schvarcz, C.R. Making sense of virus size and the tradeoffs shaping viral fitness. Ecol. Lett. 2021, 24, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Louten, J. Chapter 2—Virus Structure and Classification. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–29. [Google Scholar] [CrossRef]
- Fermin, G. Host Range, Host–Virus Interactions, and Virus Transmission. Viruses 2018, 2018, 101–134. [Google Scholar] [CrossRef]
- Fischer, M.G. The Virophage Family Lavidaviridae. Curr. Issues Mol. Biol. 2021, 40, 1–24. [Google Scholar] [CrossRef]
- Jolly, C.; Sattentau, Q.J. Attachment Factors. Adv. Exp. Med. Biol. 2006, 790, 1–23. [Google Scholar] [CrossRef]
- Karst, S.M. Pathogenesis of Noroviruses, Emerging RNA Viruses. Viruses 2010, 2, 748–781. [Google Scholar] [CrossRef]
- Ryu, W. Virus Life Cycle. Mol. Virol. Hum. Pathog. Viruses 2017, 2017, 31–45. [Google Scholar] [CrossRef]
- Payet, J.P.; Suttle, C.A. To kill or not to kill: The balance between lytic and lysogenic viral infection is driven by trophic status. Limnol. Oceanogr. 2013, 58, 465–474. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Conley, L.; Tao, Y.; Henry, A.; Koepf, E.; Cecchini, D.; Pieracci, J.; Ghose, S. Evaluation of eco-friendly zwitterionic detergents for enveloped virus inactivation. Biotechnol. Bioeng. 2017, 114, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Shieh, Y.C.; Wang, R.R. Features of material surfaces affecting virus adhesion as determined by nanoscopic quantification. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125109. [Google Scholar] [CrossRef]
- Atmar, R.L.; Ramani, S.; Estes, M.K. Human noroviruses: Recent advances in a 50-year history. Curr. Opin. Infect. Dis. 2018, 31, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; De Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Matsushima, Y.; Nagasawa, K.; Aso, J.; Saraya, T.; Yoshihara, K.; Murakami, K.; Motoya, T.; Ryo, A.; Kuroda, M.; et al. Molecular Evolution of the Protease Region in Norovirus Genogroup II. Front. Microbiol. 2019, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; De Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Corrigendum: Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2020, 101, 893. [Google Scholar] [CrossRef]
- Ramani, S.; Atmar, R.L.; Estes, M.K. Epidemiology of human noroviruses and updates on vaccine development. Curr. Opin. Gastroenterol. 2014, 30, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Wu, Q.; Cai, W.; Zhang, J.; Guo, W. Molecular characterization of new emerging GII.17 norovirus strains from South China. Infect. Genet. Evol. 2016, 40, 1–7. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Zhen, S.-S.; Wang, J.-X.; Zhang, C.-J.; Qiu, C.; Wang, S.-M.; Jiang, X.; Wang, X.-Y. Burden of acute gastroenteritis caused by norovirus in China: A systematic review. J. Infect. 2017, 75, 216–224. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Lappalainen, S.; Uusi-Kerttula, H.; Vesikari, T.; Blazevic, V. Genotype Considerations for Virus-like Particle-Based Bivalent Norovirus Vaccine Composition. Clin. Vaccine Immunol. 2015, 22, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.I.; Atmar, R.L.; Lyon, G.M.; Treanor, J.J.; Chen, W.H.; Jiang, X.; Vinje, J.; Gregoricus, N.; Frenck, R.W., Jr.; Moe, C.L.; et al. Norovirus Vaccine against Experimental Human GII.4 Virus Illness: A Challenge Study in Healthy Adults. J. Infect. Dis. 2015, 211, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.; Sherwood, J.; Cramer, J.P.; Le Cam Bouveret, N.; Lin, S.; Baehner, F.; Borkowski, A.; The NOR-204 Investigators. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine 2020, 38, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, K.; Lappalainen, S.; Huhti, L.; Vesikari, T.; Blazevic, V. Trivalent Combination Vaccine Induces Broad Heterologous Immune Responses to Norovirus and Rotavirus in Mice. PLoS ONE 2013, 8, e70409. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.; Combe, M.; Torres-Puente, M.; Garijo, R.; Guix, S.; Buesa, J.; Rodriguez-Diaz, J.; Sanjuán, R. Human norovirus hyper-mutation revealed by ultra-deep sequencing. Infect. Genet. Evol. 2016, 41, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Sala, M.R.; Arias, C.; Domínguez, A.; Bartolomé, R.; Muntada, J.M. Foodborne outbreak of gastroenteritis due to Norovirus and Vibrio parahaemolyticus. Epidemiol. Infect. 2009, 137, 626–629. [Google Scholar] [CrossRef]
- Zuckerman, A. Chapter 70, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Brown, E.A.; Day, S.P.; Jansen, R.W.; Lemon, S.M. The 5’ nontranslated region of hepatitis A virus RNA: Secondary structure and elements required for translation in vitro. J. Virol. 1991, 65, 5828–5838. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.V.; Tada, H.; von der Helm, K.; Wissel, T.; Kiehn, R.; Wimmer, E.; Deinhardt, F. The entire nucleotide sequence of the genome of human hepatitis A virus (isolate MBB). Virus Res. 1987, 8, 153–171. [Google Scholar] [CrossRef]
- Konduru, K.; Nakamura, S.M.; Kaplan, G.G. Hepatitis A virus (HAV) packaging size limit. Virol. J. 2009, 6, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, D.; Couturier, E.; Mackiewicz, V.; Graube, A.; Letort, M.-J.; Dussaix, E.; Roque-Afonso, A.-M. Epidemiology and Genetic Characterization of Hepatitis A Virus Genotype IIA. J. Clin. Microbiol. 2010, 48, 3306–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, S.M.; Jansen, R.W.; Brown, E.A. Genetic, antigenic and biological differences between strains of hepatitis A virus. Vaccine 1992, 10 (Suppl. 1), S40–S44. [Google Scholar] [CrossRef]
- Sagliocca, L.; Amoroso, P.; Stroffolini, T.; Adamo, B.; Tosti, M.E.; Lettieri, G.; Esposito, C.; Buonocore, S.; Pierri, P.; Mele, A. Efficacy of hepatitis A vaccine in prevention of secondary hepatitis A infection: A randomised trial. Lancet 1999, 353, 1136–1139. [Google Scholar] [CrossRef]
- Keeffe, E.B.; Iwarson, S.; McMahon, B.J.; Lindsay, K.L.; Koff, R.S.; Manns, M.; Baumgarten, R.; Wiese, M.; Fourneau, M.; Safary, A.; et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998, 27, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Thoelen, S.; Cramm, M.; de Groote, K.; Safary, A.; Meheus, A. Inactivated hepatitis A vaccine: Reactogenicity, immunogenicity, and long-term antibody persistence. J. Med Virol. 1994, 44, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Majumdar, M.; Thapa, B.R.; Gupta, P.K.; Khurana, J.; Budhathoki, B.; Ratho, R.K. Molecular characterization of hepatitis A virus strains in a tertiary care health set up in north western India. Indian J. Med. Res. 2015, 141, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Franco, E.; Meleleo, C.; Serino, L.; Sorbara, D.; Zaratti, L. Hepatitis A: Epidemiology and prevention in developing countries. World J. Hepatol. 2012, 4, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Collier, M.G.; Xu, F. Hepatitis A Outbreaks in Developed Countries: Detection, Control, and Prevention. Foodborne Pathog. Dis. 2020, 17, 166–171. [Google Scholar] [CrossRef]
- Gould, L.H.; Kline, J.; Monahan, C.; Vierk, K. Outbreaks of Disease Associated with Food Imported into the United States, 1996–2014(1). Emerg. Infect. Dis. 2017, 23, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Webb, G.W.; Dalton, H.R. Hepatitis E: An underestimated emerging threat. Ther. Adv. Infect. Dis. 2019, 6, 2049936119837162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, L.; DiCaprio, E. Hepatitis E Virus: An Emerging Foodborne Pathogen. Front. Sustain. Food Syst. 2018, 2, 14. [Google Scholar] [CrossRef]
- Yugo, D.M.; Meng, X.-J. Hepatitis E Virus: Foodborne, Waterborne and Zoonotic Transmission. Int. J. Environ. Res. Public Health 2013, 10, 4507–4533. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent Hepatitis E Virus Contamination in Food Containing Raw Pork Liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Ozga, A.-K.; Westhoelter, D.; Hartl, J.; Manthey, C.F.; Lütgehetmann, M.; Rauch, G.; Kriston, L.; Lohse, A.W.; Bendall, R.; et al. Hepatitis E seroprevalence in the Americas: A systematic review and meta-analysis. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.P. Critical Review of Norovirus Surrogates in Food Safety Research: Rationale for Considering Volunteer Studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, J.; Rivera-Aban, M.; Greening, G. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J. Appl. Microbiol. 2009, 107, 65–71. [Google Scholar] [CrossRef]
- Rzeżutka, A.; Cook, N. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 2004, 28, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, A.; Haines, J.; Stals, A.; Li, D.; Uyttendaele, M.; Knight, A.; Jaykus, L.-A. A systematic review of human norovirus survival reveals a greater persistence of human norovirus RT-qPCR signals compared to those of cultivable surrogate viruses. Int. J. Food Microbiol. 2016, 216, 40–49. [Google Scholar] [CrossRef]
- Yeargin, T.; Buckley, D.; Fraser, A.; Jiang, X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am. J. Infect. Control. 2016, 44, 1365–1373. [Google Scholar] [CrossRef]
- Farkas, T.; Cross, R.W.; Hargitt, E.; Lerche, N.W.; Morrow, A.L.; Sestak, K. Genetic Diversity and Histo-Blood Group Antigen Interactions of Rhesus Enteric Caliciviruses. J. Virol. 2010, 84, 8617–8625. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Yang, D.; Quigley, C.; Chou, M.; Jiang, X. Inactivation of the Tulane Virus, a Novel Surrogate for the Human Norovirus. J. Food Prot. 2013, 76, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Wei, C.; Huang, P.; Fan, Q.; Quigley, C.; Xia, M.; Fang, H.; Zhang, X.; Zhong, W.; Klassen, J.S.; et al. Tulane virus recognizes sialic acids as cellular receptors. Sci. Rep. 2015, 5, 11784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shea, H.; Blacklaws, B.A.; Collins, P.J.; McKillen, J.; Fitzgerald, R. Viruses Associated With Foodborne Infections. Ref. Modul. Life Sci. 2019. [Google Scholar] [CrossRef]
- Sair, A.I.; D’Souza, D.H.; Jaykus, L.A. Human Enteric Viruses as Causes of Foodborne Disease. Compr. Rev. Food Sci. Food Saf. 2002, 1, 73–89. [Google Scholar] [CrossRef]
- Rivadulla, E.; Romalde, J.L. A Comprehensive Review on Human Aichi Virus. Virol. Sin. 2020, 35, 501–516. [Google Scholar] [CrossRef]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive Comparison of Cultivable Norovirus Surrogates in Response to Different Inactivation and Disinfection Treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardell, D. Survival of Herpes Simplex Virus Type 1 on Some Common Foods Routinely Touched before Consumption. J. Food Prot. 1997, 60, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.M.V. Experiments on the action of light on Bacillus anthracis. Proc. R. Soc. Lond. 1893, 52, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Meechan, P.J.; Wilson, C. Use of Ultraviolet Lights in Biological Safety Cabinets: A Contrarian View. Appl. Biosaf. 2006, 11, 222–227. [Google Scholar] [CrossRef]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Berg, M.; Bergman, B.; Hoborn, J. Ultraviolet radiation compared to an ultra-clean air enclosure. Comparison of air bacteria counts in operating rooms. J. Bone Jt. Surgery 1991, 73, 811–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Allende, A.; Lopez-Galvez, F.; Conesa, M.A.; Gil, M.I. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry—A critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Lytle, C.D.; Sagripanti, J.-L. Predicted Inactivation of Viruses of Relevance to Biodefense by Solar Radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, P.; Hope, A. Virus inactivation by high intensity broad spectrum pulsed light. J. Virol. Methods 2003, 110, 61–65. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Butot, S.; Cantergiani, F.; Moser, M.; Jean, J.; Lima, A.; Michot, L.; Putallaz, T.; Stroheker, T.; Zuber, S. UV-C inactivation of foodborne bacterial and viral pathogens and surrogates on fresh and frozen berries. Int. J. Food Microbiol. 2018, 275, 8–16. [Google Scholar] [CrossRef]
- Linden, K.G.; Thurston, J.; Schaefer, R.; Malley, J.P. Enhanced UV Inactivation of Adenoviruses under Polychromatic UV Lamps. Appl. Environ. Microbiol. 2007, 73, 7571–7574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffman, D.E.; Gennaccaro, A.; Rose, J.B.; Dussert, B.W. Low- and medium-pressure UV inactivation of microsporidia Encephalitozoon intestinalis. Water Res. 2002, 36, 3161–3164. [Google Scholar] [CrossRef]
- Sholtes, K.; Linden, K.G. Pulsed and continuous light UV LED: Microbial inactivation, electrical, and time efficiency. Water Res. 2019, 165, 114965. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D. Ultraviolet Light Fights New Virus. Engineering 2020, 6, 851–853. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Smart, W.D. Surface Disinfection using Ultraviolet Lightwith a Mobile Manipulation Robot. arXiv 2021, arXiv:2104.10739. [Google Scholar]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. Conf. Res. Work. Anim. Dis. 2011, 12, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Bicalho, M.; Machado, V.; Lima, S.; Teixeira, A.; Warnick, L.; Bicalho, R. Evaluation of the effects of ultraviolet light on bacterial contaminants inoculated into whole milk and colostrum, and on colostrum immunoglobulin G. J. Dairy Sci. 2014, 97, 2866–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauth, A.M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys. J. 1965, 5, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D. DNA Repair and Mutagenesis; American Society for Microbiology Press: Washington, DC, USA, 1995; p. 698. [Google Scholar]
- Murphy, T.M.; Gordon, M.P. Photobiology of RNA Viruses; Springer: Boston, MA, USA, 1981; pp. 285–351. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-S. Inactivation of Viruses on Surfaces by Ultraviolet Germicidal Irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Plagemann, P.G.W. Effect of Ultraviolet Light on Mengovirus: Formation of Uracil Dimers, Instability and Degradation of Capsid, and Covalent Linkage of Protein to Viral RNA. J. Virol. 1974, 13, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Brickner, P.W.; Vincent, R.L.; First, M.; Nardell, E.; Murray, M.; Kaufman, W. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: Bioterrorism countermeasure. Public Health Rep. 2003, 118, 99–114. [Google Scholar] [CrossRef]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Comparison of UV-Induced Inactivation and RNA Damage in MS2 Phage across the Germicidal UV Spectrum. Appl. Environ. Microbiol. 2015, 82, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat. Res. 2017, 187, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-W.; Matsuura, R.; Iimura, K.; Wada, S.; Shinjo, A.; Benno, Y.; Nakagawa, M.; Takei, M.; Aida, Y. UVC disinfects SARS-CoV-2 by induction of viral genome damage without apparent effects on viral morphology and proteins. Sci. Rep. 2021, 11, 13804. [Google Scholar] [CrossRef]
- Rezaie, A.; Leite, G.G.S.; Melmed, G.Y.; Mathur, R.; Villanueva-Millan, M.J.; Parodi, G.; Sin, J.; Germano, J.F.; Morales, W.; Weitsman, S.; et al. Ultraviolet A light effectively reduces bacteria and viruses including coronavirus. PLoS ONE 2020, 15, e0236199. [Google Scholar] [CrossRef] [PubMed]
- Eischeid, A.C.; Linden, K.G. Molecular Indications of Protein Damage in Adenoviruses after UV Disinfection. Appl. Environ. Microbiol. 2011, 77, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuanualsuwan, S.; Cliver, D.O. Capsid Functions of Inactivated Human Picornaviruses and Feline Calicivirus. Appl. Environ. Microbiol. 2003, 69, 350–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, S.E.; Hull, N.M.; Poepping, C.; Linden, K.G. Wavelength-Dependent Damage to Adenoviral Proteins across the Germicidal UV Spectrum. Environ. Sci. Technol. 2018, 52, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Vimont, A.; Fliss, I.; Jean, J.; Deng, Y.; Liu, X.; Wu, J.; Lee, J.; Chen, S.; Cheng, Y.; Zhang, C.; et al. Efficacy and Mechanisms of Murine Norovirus Inhibition by Pulsed-Light Technology. Appl. Environ. Microbiol. 2015, 81, 2950–2957. [Google Scholar] [CrossRef] [Green Version]
- Sancar, A. No “End of History” for Photolyases. Science 1996, 272, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Schnitzlein, W.M.; Tripathy, D.N. Fowlpox Virus Encodes a Novel DNA Repair Enzyme, CPD-Photolyase, That Restores Infectivity of UV Light-Damaged Virus. J. Virol. 2001, 75, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Jean, J.; Morales-Rayas, R.; Anoman, M.-N.; Lamhoujeb, S. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol. 2011, 28, 568–572. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Lamont, Y.; Rzeżutka, A.; Anderson, J.; MacGregor, S.; Given, M.; Deppe, C.; Cook, N. Pulsed UV-light inactivation of poliovirus and adenovirus. Lett. Appl. Microbiol. 2007, 45, 564–567. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, M.; Cao, X.; Chen, H. Pulsed light inactivation of murine norovirus, Tulane virus, Escherichia coli O157:H7 and Salmonella in suspension and on berry surfaces. Food Microbiol. 2017, 61, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Belliot, G.; Loutreul, J.; Estienney, M.; Cazeaux, C.; Nicorescu, I.; Aho, S.; Gervais, P.; Orange, N.; Pothier, P.; Morin, T. Potential of Pulsed Light to Inactivate Bacteriophage MS2 in Simple Liquid Medium and on Complex Foodstuffs. Food Environ. Virol. 2013, 5, 176–179. [Google Scholar] [CrossRef]
- Emmoth, E.; Rovira, J.; Rajkovic, A.; Corcuera, E.; Pérez, D.W.; Dergel, I.; Ottoson, J.R.; Widén, F. Inactivation of Viruses and Bacteriophages as Models for Swine Hepatitis E Virus in Food Matrices. Food Environ. Virol. 2017, 9, 20–34. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H. Inactivation of Escherichia coli O157:H7, Salmonella and human norovirus surrogate on artificially contaminated strawberries and raspberries by water-assisted pulsed light treatment. Food Res. Int. 2015, 72, 1–7. [Google Scholar] [CrossRef]
- Bhullar, M.; Patras, A.; Kilonzo-Nthenge, A.; Pokharel, B.; Sasges, M. Ultraviolet inactivation of bacteria and model viruses in coconut water using a collimated beam system. Food Sci. Technol. Int. 2019, 25, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, M.; Patras, A.; Kilanzo-Nthenge, A.; Pokharel, B.; Yannam, S.K.; Rakariyatham, K.; Pan, C.; Xiao, H.; Sasges, M. Microbial inactivation and cytotoxicity evaluation of UV irradiated coconut water in a novel continuous flow spiral reactor. Food Res. Int. 2018, 103, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gunter-Ward, D.M.; Patras, A.; Bhullar, M.; Kilonzo-Nthenge, A.; Pokharel, B.; Sasges, M. Efficacy of ultraviolet (UV-C) light in reducing foodborne pathogens and model viruses in skim milk. J. Food Process Preserv. 2018, 42, e13485. [Google Scholar] [CrossRef]
- Ward, D.M.; Patras, A.; Kilonzo-Nthenge, A.; Yannam, S.K.; Pan, C.; Xiao, H.; Sasges, M. UV-C treatment on the safety of skim milk: Effect on microbial inactivation and cytotoxicity evaluation. J. Food Process Eng. 2019, 42, e12944. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 18, 464–473. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Ultraviolet-C Radiation on the Fresh Chicken Breast: Inactivation of Major Foodborne Viruses and Changes in Physicochemical and Sensory Qualities of Product. Food Bioprocess Technol. 2015, 8, 895–906. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Kniel, K.E. Inactivation of internalized and surface contaminated enteric viruses in green onions. Int. J. Food Microbiol. 2013, 166, 201–206. [Google Scholar] [CrossRef]
- Fino, V.R.; Kniel, K.E. UV Light Inactivation of Hepatitis A Virus, Aichi Virus, and Feline Calicivirus on Strawberries, Green Onions, and Lettuce. J. Food Prot. 2008, 71, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Solà, J.; Viñas, I.; Aguiló-Aguayo, I.; Bobo, G.; Abadias, M. An innovative water-assisted UV-C disinfection system to improve the safety of strawberries frozen under cryogenic conditions. Innov. Food Sci. Emerg. Technol. 2021, 73, 102756. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, A.-N.; Lee, K.-H.; Ha, S.-D. Ultraviolet-C efficacy against a norovirus surrogate and hepatitis A virus on a stainless steel surface. Int. J. Food Microbiol. 2015, 211, 73–78. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Chen, H. Application of water-assisted ultraviolet light processing on the inactivation of murine norovirus on blueberries. Int. J. Food Microbiol. 2015, 214, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Han, S.; Son, J.W.; Park, S.H.; Ha, S.-D. Impact of ultraviolet-C and peroxyacetic acid against murine norovirus on stainless steel and lettuce. Food Control. 2021, 130, 108378. [Google Scholar] [CrossRef]
- Xie, Y.; Hajdok, C.; Mittal, G.S.; Warriner, K. Inactivation of MS2 F(+) Coliphage on Lettuce by a Combination of UV Light and Hydrogen Peroxide. J. Food Prot. 2008, 71, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Nasheri, N.; Harlow, J.; Chen, A.; Corneau, N.; Bidawid, S. Survival and Inactivation by Advanced Oxidative Process of Foodborne Viruses in Model Low-Moisture Foods. Food Environ. Virol. 2021, 13, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef]
- Zoschke, K.; Börnick, H.; Worch, E. Vacuum-UV radiation at 185 nm in water treatment—A review. Water Res. 2014, 52, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Jubinville, E.; Girard, M.; Trudel-Ferland, M.; Fliss, I.; Jean, J. Inactivation of Murine Norovirus Suspended in Organic Matter Simulating Actual Conditions of Viral Contamination. Food Environ. Virol. 2021, 13, 544–552. [Google Scholar] [CrossRef]
- Berry, N.L.; Overholt, E.P.; Fisher, T.J.; Williamson, C.E. Dissolved organic matter protects mosquito larvae from damaging solar UV radiation. PLoS ONE 2020, 15, e0240261. [Google Scholar] [CrossRef]
- McClain, M.E.; Spendlove, R.S. Multiplicity Reactivation of Reovirus Particles after Exposure to Ultraviolet Light. J. Bacteriol. 1966, 92, 1422–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurston-Enriquez, J.A.; Haas, C.; Jacangelo, J.; Riley, K.; Gerba, C.P. Inactivation of Feline Calicivirus and Adenovirus Type 40 by UV Radiation. Appl. Environ. Microbiol. 2003, 69, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pexara, A.; Govaris, A. Foodborne Viruses and Innovative Non-Thermal Food-Processing Technologies. Foods 2020, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Goswami, B. Pathogenic mechanisms of foodborne viral disease. Food Consum. Dis. Risk 2006, 2006, 343. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Code of Federal Regulations. Title 21, Volume 3, 21CFR179.39. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=179.39 (accessed on 1 November 2021).
- Koutchma, T. Status of International Regulations for Ultraviolet Treatment of Foods. IUVA News 2018, Quarter 2, 14–16. Available online: https://uvsolutionsmag.com/stories/pdf/IUVA_2018_Quarter2_full_Koutchma_links.pdf (accessed on 1 November 2021).
- U.S. Food & Drug Administration. Code of Federal Regulations. Title 21, Volume 3, 21CFR179.41. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=179.41 (accessed on 1 November 2021).
- U.S. Food & Drug Administration. Code of Federal Regulations. Title 21, Volume 3, 21CFR172.381. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.381 (accessed on 1 November 2021).
- European Food Safety Authority (EFSA). Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2470&from=EN (accessed on 1 November 2021).
- Canada, H. Ultraviolet Light Treatment of Apple Juice/Cider Using the CiderSure 3500. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/novel-food-information-ultraviolet-light-treatment-apple-juice-cider-using-cidersure-3500.html (accessed on 1 November 2021).
| Virus | Matrix | Fluence (J/cm2) | Log Inactivation | References |
|---|---|---|---|---|
| Adenovirus | PBS * | 5.6 | 4.0 | [145] |
| Bovine parvovirus | PBS | 1.0 | 4.3 | [117] |
| Canine parvovirus | PBS | 1.0 | >6.5 | [117] |
| Encephalomyocarditis | PBS | 1.0 | >5.9 | [117] |
| Escherichia coli phage MS2 | Black pepper | 9.4 | 0.64 | [147] |
| Garlic | 18.8 | 0.40 | ||
| Chopped mint | 18.8 | 1.28 | ||
| Glass beads | 9.4 | 4.87 | ||
| PBS | 3.8 | >8 | ||
| Swine liver | 60 | 1.6 | [148] | |
| Ham | 60 | 0.97 | ||
| Sausage | 60 | 1.3 | ||
| HAV | PBS | >5.7 | [117] | |
| PBS | 0.05 | 4.8 | [143] | |
| Stainless steel | 0.06 | 5 | ||
| PVC | 0.091 | 5 | ||
| HSV-1 | PBS | 1.0 | >4.8 | [117] |
| MNV-1 | Strawberry | 1.27 | 1.8 | [149] |
| Raspberries | 1.27 | 3.6 | ||
| PVC | 2.07 | 3 | [140] | |
| Blueberry | 22.5 | 3.8 | [146] | |
| Strawberry | 22.5 | 0.9 | ||
| PBS | 2.47 | 5.8 | ||
| PBS | 0.06 | 5.0 | [143] | |
| PVC | 5 | |||
| Stainless steel | 0.06 | 5 | ||
| PBS | 2.07 | 3.3 | [140] | |
| Alginate | 0.69 | 3.6 | ||
| Hard water | 4.84 | 3.9 | ||
| Turbid water | 3.45 | 3 | ||
| Stainless steel | 8.98 | 2.6 | ||
| Phage φX174 | Swine Liver | 60 | 2 | [148] |
| Ham | 60 | 1.6 | ||
| Sausage | 60 | 1.6 | ||
| Poliovirus | PBS | 0.28 | 4.0 | [145] |
| 1 | >6.7 | [117] | ||
| Simian virus 40 | PBS | 1.0 | 3.7 | [117] |
| Sindbis | PBS | 1.0 | 7.2 | [117] |
| TV | PBS | 4.94 | 6 | [146] |
| Vaccinia | PBS | 1.0 | >5.1 | [117] |
| Virus | Substrate | Fluence (J/cm2) for 1 log Reduction | Fluence (J/cm2) for 5 log Reduction | References |
|---|---|---|---|---|
| Adenovirus type 41 | Green onions | 0.240 (3 log) * | [156] | |
| Aichi virus | Romaine lettuce | 0.240 | [157] | |
| Green onions | 0.240 (3.66 log) * | |||
| Strawberries | 0.240 | |||
| FCV | Romaine lettuce | 0.240 | ||
| Green onions | 0.240 (3.92 logs) * | |||
| Strawberries | 0.240 (2.28 log) * | |||
| HAV | Frozen strawberries | 0.212 | [116] | |
| 0.13 | [158] | |||
| Frozen blueberries | 0.212 | [116] | ||
| Fresh strawberries | 0.212 | |||
| 0.240 (2.60 log) * | [157] | |||
| Fresh blueberries | 0.212 | |||
| Fresh raspberries | 0.212 | [159] | ||
| Romaine lettuce | 0.240 | [157] | ||
| Green onions | 0.240 | |||
| 0.240 | [156] | |||
| MNV-1 | Fresh blueberry | 1.331 | [116] | |
| Fresh blueberry | 1.2 (Dry) | 1.2 (Water-assisted) | [160] | |
| Green onions | 0.24 | [156] | ||
| Lettuce | 0.6 | [161] | ||
| Phage MS2 | Iceberg Lettuce | 0.019 | [162] |
| Virus | Substrate | Fluence (J/cm2) for 1 log Reduction | Fluence (J/cm2) for 5 log Reduction | References |
|---|---|---|---|---|
| FCV | Chocolate | 3.8 (4 log) * | [163] | |
| Pistachios | 3.8 (2 log) * | |||
| Cornflakes | 3.8 | |||
| HAV | Fresh chicken breast Stainless steel surface Chocolate Pistachios Cornflakes | 3.6 0.3 3.8 (2 logs) * 3.8 3.8 | [155] [163] | |
| MNV-1 | Fresh chicken breast | 3.6 | [155] | |
| Stainless steel surface | 0.3 | |||
| 1.8 (2.65 log) * | [161] | |||
| Chocolate | 3.8 (4 log) * | [163] | ||
| Pistachios | 3.8 (1.5 log) * | |||
| Cornflakes | 3.8 (0.6 log) * | |||
| Phage MS2 | Skim milk | 0.150 | [152] | |
| 0.168 | [153] | |||
| Coconut water | 0.02 | 0.12(4.20 log) * | [151] | |
| 0.1 | [150] | |||
| Phage T1 | Skim milk | 0.0062 | [152] | |
| 0.027 | [153] | |||
| Coconut water | 0.005 | 0.03 (4.73 log) * | [151] | |
| 0.03 | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, V.M.; Jubinville, E.; Rodríguez-López, M.I.; Trudel-Ferland, M.; Bouchard, S.; Jean, J. Inactivation of Foodborne Viruses by UV Light: A Review. Foods 2021, 10, 3141. https://doi.org/10.3390/foods10123141
Gómez-López VM, Jubinville E, Rodríguez-López MI, Trudel-Ferland M, Bouchard S, Jean J. Inactivation of Foodborne Viruses by UV Light: A Review. Foods. 2021; 10(12):3141. https://doi.org/10.3390/foods10123141
Chicago/Turabian StyleGómez-López, Vicente M., Eric Jubinville, María Isabel Rodríguez-López, Mathilde Trudel-Ferland, Simon Bouchard, and Julie Jean. 2021. "Inactivation of Foodborne Viruses by UV Light: A Review" Foods 10, no. 12: 3141. https://doi.org/10.3390/foods10123141
APA StyleGómez-López, V. M., Jubinville, E., Rodríguez-López, M. I., Trudel-Ferland, M., Bouchard, S., & Jean, J. (2021). Inactivation of Foodborne Viruses by UV Light: A Review. Foods, 10(12), 3141. https://doi.org/10.3390/foods10123141