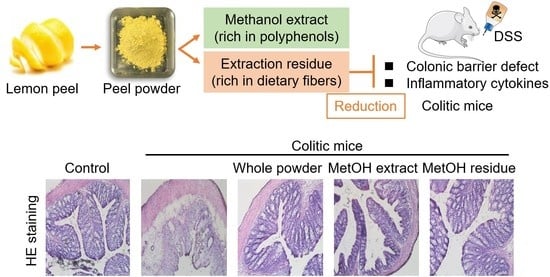
Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Citrus Limon Peel Powder and Fractions
2.3. Nutritional Analyses of Citrus limon Peel Powder and the Two Fractions
2.4. Animals and Diets
2.5. Experimental Design
2.6. Colitis Clinical Score
2.7. Histopathology
2.8. Immunoblot Analysis
2.9. Immunofluorescence
2.10. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.11. Fecal Organic Acid Analysis
2.12. Statistical Analysis
3. Results
3.1. Nutritional Characterization of Whole LP Powder and Its Fractions
3.2. Effects of Whole LP Powder and Its Fractions on Bodyweight Loss and Colitis Clinical Score
3.3. Effect of Whole LP Powder and Its Fractions on the Colonic TJ Barrier
3.4. Effect of Whole LP Powder and Its Fractions on Colonic Gene Expression
3.5. Effect of Whole LP Powder and Its Fractions on Fecal Organic Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanauer, S.B. Inflammatory bowel disease. N. Engl. J. Med. 1996, 334, 841–848. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Rao, R.K.; Basuroy, S.; Rao, V.U.; Karnaky Jr, K.J.; Gupta, A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002, 368, 471–481. [Google Scholar] [CrossRef]
- Suzuki, T.; Seth, A.; Rao, R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J. Biol. Chem. 2008, 283, 3574–3583. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Mayangsari, Y.; Suzuki, T. Resveratrol ameliorates intestinal barrier defects and inflammation in colitic mice and intestinal cells. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Tanabe, S.; Suzuki, T. Naringenin enhances intestinal barrier function through the expression and cytoskeletal association of tight junction proteins in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yasui, Y.; Ishigamori-Suzuki, R.; Oyama, T. Citrus compounds inhibit inflammation- and obesity-related colon carcinogenesis in mice. Nutr. Cancer 2008, 60 (Suppl. 1), 70–80. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Van Hung, T.; Nagata, Y.; Fukuda, N.; Suzuki, T. Citrus kawachiensis peel powder reduces intestinal barrier defects and inflammation in colitic mice. J. Agric. Food Chem. 2018, 66, 10991–10999. [Google Scholar] [CrossRef]
- Del Río, J.A.; Fustera, M.D.; Gómeza, P.; Porras, I.; García-Lidón, A.; Ortuño, A. Citrus limon: A source of flavonoids of pharmaceutical interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Nagashio, Y.; Matsuura, Y.; Miyamoto, J.; Kometani, T.; Suzuki, T.; Tanabe, S. Hesperidin inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th17 activity. J. Funct. Foods 2013, 5, 1633–1641. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Z.L.; Li, P.; Zhou, Y.Q. Modulating effect of Hesperidin on experimental murine colitis induced by dextran sulfate sodium. Phytomedicine 2009, 16, 989–995. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.A.; et al. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Choi, M.S. Eriocitrin improves adiposity and related metabolic disorders in high-fat diet-induced obese mice. J. Med. Food 2020, 23, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funaguchi, N.; Ohno, Y.; La, B.L.; Asai, T.; Yuhgetsu, H.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 766–770. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Dietary Fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2016, 146, 1970–1979. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J. Nutr. 2018, 148, 552–561. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, L.; Garris, S.T.; Morgan, J.N. Comparison of five extraction methods for determination of incurred and added pesticides in dietary composites. J. AOAC Int. 2002, 85, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [Green Version]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Okuma, K. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Chaen, Y.; Yamamoto, Y.; Suzuki, T. Naringenin promotes recovery from colonic damage through suppression of epithelial tumor necrosis factor-alpha production and induction of M2-type macrophages in colitic mice. Nutr. Res. 2019, 64, 82–92. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, U. Medical treatment of ulcerative colitis. Clin. Colon Rectal Surg. 2004, 17, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Ishioka, M.; Fujita, Y.; Umeno, A.; Yasunaga, M.; Sato, A.; Ohnishi, S.; Suzuki, S.; Ishida, N.; Shichiri, M.; et al. Yuzu (Citrus junos Tanaka) Peel attenuates dextran sulfate sodium-induced murine experimental colitis. J. Oleo Sci. 2018, 67, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, M.T.; Vezza, T.; Diez-Echave, P.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef] [Green Version]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain Fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, W.; Wu, X.; Wang, K.; Wang, W.; Wang, Y.; Li, Z.; Liu, J.; Li, L.; Peng, L. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCbeta2. Int. J. Mol. Sci. 2016, 17, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.V.; Suzuki, T. Short-chain fatty acids suppress inflammatory reactions in Caco-2 cells and mouse colons. J. Agric. Food Chem. 2018, 66, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Toyonaga, A.; Sasaki, E.; Watanabe, K.; Tateishi, H.; Nishiyama, T.; Saiki, T.; Ikeda, H.; Tsuruta, O.; Tanikawa, K. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1994, 96, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Baethge, B.A.; Wolf, R.E.; Betzing, K.W.; Stewart, R.M.; Hall, V.C.; Fukuda, M. Correlation of serum interleukin-8 and cell surface lysosome-associated membrane protein expression with clinical disease activity in systemic lupus erythematosus. Lupus 1994, 3, 97–102. [Google Scholar] [CrossRef]
- Farooq, S.M.; Stillie, R.; Svensson, M.; Svanborg, C.; Strieter, R.M.; Stadnyk, A.W. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J. Pharmacol. Exp. Ther. 2009, 329, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Macfarlane, G.T.; Macfarlane, S. Intestinal bacteria and ulcerative colitis. Curr. Issues Intest. Microbiol. 2003, 4, 9–20. [Google Scholar]
- De Musis, C.; Granata, L.; Dallio, M.; Miranda, A.; Gravina, A.G.; Romano, M. Inflammatory bowel diseases: The role of gut microbiota. Curr. Pharm. Des. 2020, 26, 2951–2961. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, D.H.; Jiang, M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 2012, 29, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1140–G1149. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.P.; Pereira, I.A.; Mougenel, J.L.; Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Guo, G.; Shi, W.; Shi, F.; Gong, W.; Li, F.; Zhou, G.; She, J. Anti-inflammatory effects of eriocitrin against the dextran sulfate sodium-induced experimental colitis in murine model. J. Biochem. Mol. Toxicol. 2019, 33, e22400. [Google Scholar] [CrossRef]
- Guo, K.; Ren, J.; Gu, G.; Wang, G.; Gong, W.; Wu, X.; Ren, H.; Hong, Z.; Li, J. Hesperidin protects against intestinal inflammation by restoring intestinal barrier function and up-regulating Treg cells. Mol. Nutr. Food Res. 2019, 63, e1800975. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [Green Version]







| Ingredient | Control Diet |
|---|---|
| g/kg diet | |
| Casein b | 200 |
| α-Corn starch c | 529.5 |
| Sucrose | 100 |
| Soybean oil | 70 |
| Choline bitartrate | 2.5 |
| l-Cystine | 3 |
| Mineral mixture d | 35 |
| Vitamin mixture d | 10 |
| Cellulose e | 50 |
| Score | Diarrhea Stool | Bloody Stool | Weight Loss (% of Initial) |
|---|---|---|---|
| 0 | Normal | Normal color | <1 |
| 1 | Mildly soft | Brown color | 1–5 |
| 2 | Very soft | Reddish color | 6–10 |
| 3 | Watery | Bloody stool | 11–20 |
| 4 | More watery | More bloody | >21 |
| Target Gene | Forward | Reverse |
|---|---|---|
| Mouse Il6 | 5′-CTGATGCTGGTGACAACCAC-3′ | 5′-TCCACGATTTCCCAGAGAAC-3′ |
| Mouse Il17a | 5′-AGCTGGACCACCACTTGAAT-3′ | 5′-ACACCCACCAGCATCTTCTC-3′ |
| Mouse Ccl2 | 5′-GGAATGGGTCCAGACATACATTA-3′ | 5′-TAGCTTCAGATTTACGGGTCAAC-3′ |
| Mouse Cxcl2 | 5′-AGTGAACTGCGCTGTCAATG-3′ | 5′-ACTTTTTGACCGCCCTTGAG-3′ |
| Mouse Rpl13 | 5′-TCAAGAAGGTGGTGAAGCAG-3′ | 5′-AAGGTGGAAGAGTGGGAGTTG-3′ |
| Whole LP Powder | MetOH Extract | MetOH Extraction Residue | |
|---|---|---|---|
| Protein (g/100 g powder) | 0.7 | N.A. | 0.7 |
| Lipid (g/100 g powder) | 4.3 | N.A. | 5.8 |
| Ash (g/100 g powder) | 3.9 | N.A. | 4.0 |
| Dietary fiber (g/100 g powder) | 47.1 | N.A. | 53.1 |
| Water soluble | 12.3 | N.A. | 17.8 |
| Water insoluble | 34.8 | N.A. | 35.3 |
| Moisture | 7.0 | N.A. | 9.2 |
| Polyphenols (mg/100 g powder) | |||
| Hesperidin | 427 | 423 | N.D. |
| Eriocitrin | 174 | 134 | N.D. |
| Diosmin | 146 | 130 | N.D. |
| Narirutin | 26.5 | 13.5 | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. https://doi.org/10.3390/foods10020240
Tinh NTT, Sitolo GC, Yamamoto Y, Suzuki T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods. 2021; 10(2):240. https://doi.org/10.3390/foods10020240
Chicago/Turabian StyleTinh, Nguyen Thi Thanh, Gertrude Cynthia Sitolo, Yoshinari Yamamoto, and Takuya Suzuki. 2021. "Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model" Foods 10, no. 2: 240. https://doi.org/10.3390/foods10020240
APA StyleTinh, N. T. T., Sitolo, G. C., Yamamoto, Y., & Suzuki, T. (2021). Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods, 10(2), 240. https://doi.org/10.3390/foods10020240