The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
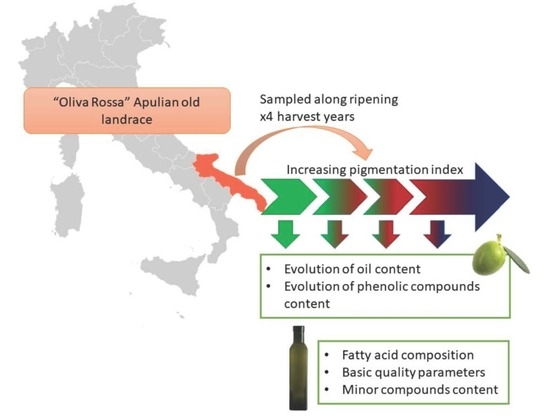
2.1. Plant Material, Sampling Plan and VOO Extraction
2.2. Drupes Analysis
2.3. VOO Analysis
2.4. Statistical Analysis
3. Results
3.1. Sampling Issues
3.2. Drupes Characteristics
3.3. VOOs Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahrburg, U.; Kratz, M.; Cullen, P. Mediterranean diet, olive oil and health. Eur. J. Lipid Sci. Technol. 2002, 104, 698–705. [Google Scholar] [CrossRef]
- UNESCO. Intergovernmental Committee for the Safeguarding of the Intangible Cultural Heritage. ITH/13/8.COM/Decisions. Decision 8.COM 8.10. Paris. 2013. Available online: https://ich.unesco.org/en/8com (accessed on 10 December 2020).
- CIHEAM/FAO. Mediterranean Food Consumption Patterns: Diet, Environment, Society, Economy and Health. Available online: http://www.fao.org/3/a-i4358e.pdf (accessed on 3 December 2020).
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef] [PubMed]
- Colomer, R.; Menéndez, J.A. Mediterranean diet, olive oil and cancer. Clin. Transl. Oncol. 2006, 8, 15–21. [Google Scholar] [CrossRef]
- Covas, M.I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007, 55, 175–186. [Google Scholar] [CrossRef]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef]
- Carbone, A.; Cacchiarelli, L.; Sabbatini, V. Exploring quality and its value in the Italian olive oil market: A panel data analysis. Agric. Food Econ. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Ilarioni, L.; Proietti, P. Olive tree cultivars. In The Extra-Virgin Olive Oil Handbook, 1st ed.; Peri, C., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; pp. 59–67. [Google Scholar]
- Rotondi, A.; Alfei, B.; Magli, M.; Pannelli, G. Influence of genetic matrix and crop year on chemical and sensory profiles of Italian monovarietal extra-virgin olive oils. J. Sci. Food Agric. 2010, 90, 2641–2648. [Google Scholar] [CrossRef]
- Di Lecce, G.; Piochi, M.; Pacetti, D.; Frega, N.G.; Bartolucci, E.; Scortichini, S.; Fiorini, D. Eleven monovarietal extra virgin olive oils from olives grown and processed under the same conditions: Effect of the cultivar on the chemical composition and sensory traits. Foods 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Brkić Bubola, K. Inter-varietal diversity of typical volatile and phenolic profiles of croatian extra virgin olive oils as revealed by GC-IT-MS and UPLC-DAD analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. Re.Ger.OP: An integrated project for the recovery of ancient and rare olive germplasm. Front. Plant. Sci. 2020, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- Squeo, G.; Difonzo, G.; Silletti, R.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Caponio, F.C. Bambina, una varietà minore pugliese: Profilo di maturazione, composizione delle drupe e caratterizzazione chimica dell’olio vergine. Riv. Ital. Sostanze Grasse 2019, 96, 143–149. [Google Scholar]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Ventre, C.; Poiana, M. Characterization of monovarietal olive oils obtained from mills of Calabria region (Southern Italy). Food Chem. 2016, 213, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of the World’s Biodiversity for Food and Agriculture; Bélanger, J., Pilling, D., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2019; Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 3 December 2020).
- Council of the European Communities. Council regulation (EEC) No 2081/92 of 14 July of 1992 and subsequent integrations and amendments. O. J. Eur. Communities 1992, 208, 1–8. [Google Scholar]
- Cacchiarelli, L.; Carbone, A.; Laureti, T.; Sorrentino, A. The value of the certifications of origin: A comparison between the Italian olive oil and wine markets. Br. Food J. 2016, 118, 824–839. [Google Scholar] [CrossRef]
- Rotondi, A.; Magli, M.; Morrone, L.; Alfei, B.; Pannelli, G. Italian National Database of Monovarietal Extra Virgin Olive Oils. In The Mediterranean Genetic Code—Grapevine and Olive; Poljuha, D., Sladonja, B., Eds.; IntechOpen: London, UK, 2013; pp. 179–200. [Google Scholar]
- Muzzalupo, I.; Lombardo, N.; Musacchio, A.; Noce, M.E.; Pellegrino, G.; Perri, E.; Sajjad, A. DNA sequence analysis of microsatellite markers enhances their efficiency for germplasm management in an Italian olive collection. J. Am. Soc. Hortic. Sci. 2006, 131, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Oli Monovarietali Italiani by ASSAM Marche and CNR-IBE. Available online: http://www.olimonovarietali.it/ (accessed on 3 December 2020).
- Oleadb: Worldwide Database for the Management of Genetic Resources of Olive (Olea europaea L.). Available online: http://www.oleadb.it/olivodb.html (accessed on 3 December 2020).
- Squeo, G.; Difonzo, G.; Summo, C.; Crecchio, C.; Caponio, F. Study of the influence of technological coadjuvants on enzyme activities and phenolic and volatile compounds in virgin olive oil by a response surface methodology approach. LWT Food Sci. Technol. 2020, 133, 109887. [Google Scholar] [CrossRef]
- Protezione Civile Regione Puglia. Available online: https://protezionecivile.puglia.it/ (accessed on 25 January 2021).
- Difonzo, G.; Fortunato, S.; Tamborrino, A.; Squeo, G.; Bianchi, B.; Caponio, F. Development of a modified malaxer reel: Influence on mechanical characteristic and virgin olive oil quality and composition. LWT Food Sci. Technol. 2021, 135, 110290. [Google Scholar] [CrossRef]
- Zago, L.; Squeo, G.; Bertoncini, E.I.; Difonzo, G.; Caponio, F. Chemical and sensory characterization of Brazilian virgin olive oils. Food Res. Int. 2019, 126, 108588. [Google Scholar] [CrossRef] [PubMed]
- Squeo, G.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Khmelinskii, I.; Sikorska, E. Evaluation of total phenolic content in virgin olive oil using fluorescence excitation–emission spectroscopy coupled with chemometrics. J. Sci. Food Agric. 2019, 99, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities. Commission regulation (EEC) No 2568/91 of 11 July of 1991 and subsequent integrations and amendments. Off. J. Eur. Communities 1991, 248, 1–83. [Google Scholar]
- Pokorny, J.; Kalinova, L.; Dysseler, P. Determination of chlorophyll pigments in crude vegetable oils: Results of a collaborative study and the standardized method (Technical Report). Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar] [CrossRef]
- Makhlouf, F.Z.; Squeo, G.; Barkat, M.; Trani, A.; Caponio, F. Antioxidant activity, tocopherols and polyphenols of acorn oil obtained from Quercus species grown in Algeria. Food Res. Int. 2018, 114, 208–213. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Squeo, G.; Pasqualone, A.; Caponio, F.; Summo, C. An easy and green tool for olive oils labelling according to the contents of hydroxytyrosol and tyrosol derivatives: Extraction with a natural deep eutectic solvent and direct spectrophotometric analysis. Food Chem. 2019, 291, 1–6. [Google Scholar] [CrossRef]
- Caponio, F.; Leone, A.; Squeo, G.; Tamborrino, A.; Summo, C. Innovative technologies in virgin olive oil extraction process: Influence on volatile compounds and organoleptic characteristics. J. Sci. Food Agric. 2019, 99, 5594–5600. [Google Scholar] [CrossRef]
- Tozzini, L. Pesano sulla produzione i danni della gelata. Olivo e Olio 2019, 1, 8–9. (In Italian) [Google Scholar]
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening indices and harvesting times of different olive cultivars for continuous harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- Trapani, S.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Canuti, V.; Fia, G.; Zanoni, B. Direct quantitative indices for ripening of olive oil fruits to predict harvest time. Eur. J. Lipid Sci. Technol. 2016, 118, 1202–1212. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Janik, J., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2011; Volume 38, pp. 83–148. [Google Scholar]
- Klen, T.J.; Vodopivec, B.M. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two-and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- European commission. Commission Regulation (EU) No 432/2012 of 16 May 2012. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Kiritsakis, A.K.; Nanos, G.D.; Polymenoupoulos, Z.; Thomai, T.; Sfakiotakis, E.Y. Effect of fruit storage conditions on olive oil quality. J. Am. Oil Chem. Soc. 1998, 75, 721–724. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food. Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Pasqualone, A. Phenolic compounds in virgin olive oils: Influence of the degree of olive ripeness on organoleptic characteristics and shelf-life. Eur. Food Res. Technol. 2001, 212, 329–333. [Google Scholar] [CrossRef]
- Beltrán, G.; Aguilera, M.P.; Del Rio, C.; Sanchez, S.; Martinez, L. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem. 2005, 89, 207–215. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Cepero, M.R.L.; Mínguez-Mosquera, M.I. Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. J. Am. Oil Chem. Soc. 2000, 77, 853–858. [Google Scholar] [CrossRef]
- Criado, M.N.; Romero, M.P.; Casanovas, M.; Motilva, M.J. Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem. 2008, 110, 873–880. [Google Scholar] [CrossRef]
- Roca, M.; Minguez-Mosquera, M.I. Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. J. Am. Oil Chem. Soc. 2001, 78, 133–138. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [Green Version]
- Caponio, F.; Squeo, G.; Brunetti, L.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Catalano, P.; Bianchi, B. Influence of the feed pipe position of an industrial scale two-phase decanter on extraction efficiency and chemical-sensory characteristics of virgin olive oil. J. Sci. Food Agric. 2018, 98, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Ilyasoglu, H.; Ozcelik, B.; Van Hoed, V.; Verhe, R. Cultivar characterization of Aegean olive oils with respect to their volatile compounds. Sci. Hortic. 2011, 129, 279–282. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.D.C. Sensory defects of virgin olive oil from a microbiological perspective. Trends Food Sci. Technol. 2015, 43, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME–GC–MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef] [PubMed]



| 2017/2018 | 2019/2020 | |||||
|---|---|---|---|---|---|---|
| Pi * | 1.92 | 1.93 | 3.12 | 2.43 | 3.57 | 3.83 |
| Fatty acid | S2 | S3 | S4 | S2 | S3 | S4 |
| C14:0 | 0.01 ± 0.00 b,c | 0.01 ± 0.00 b,c | 0.01 ± 0.00 c | 0.01 ± 0.01 c | 0.02 ± 0.00 a,b | 0.03 ± 0.01 a |
| C16:0 | 11.11 ± 0.28 b | 10.24 ± 0.02 b | 12.84 ± 0.61 a | 12.54 ± 0.28 a | 10.31 ± 0.03 b | 10.75 ± 0.80 b |
| C16:1 | 0.48 ± 0.01 c | 0.48 ± 0.00 c | 0.55 ± 0.01 c | 1.04 ± 0.07 a | 0.78 ± 0.01 b | 0.79 ± 0.10 b |
| C17:0 | 0.04 ± 0.00 d | 0.05 ± 0.00 c,d | 0.05 ± 0.00 c,d | 0.10 ± 0.01 a | 0.10 ± 0.01 a,b | 0.07 ± 0.03 b,c |
| C17:1 | 0.07 ± 0.01 a | 0.08 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.01 a | 0.05 ± 0.01 b | 0.04 ± 0.01 b |
| C18:0 | 2.75 ± 0.01 a | 2.56 ± 0.00 b,c,d | 2.47 ± 0.04 c,d | 2.46 ± 0.10 d | 2.65 ± 0.03 a,b | 2.59 ± 0.11 b,c |
| C18:1 | 73.58 ± 0.17 c | 76.21 ± 0.01 a | 74.38 ± 0.50 b | 71.83 ± 0.32 d | 70.84 ± 0.19 e | 70.73 ± 0.50 e |
| C18:2 | 10.37 ± 0.11 b | 8.80 ± 0.01 c | 8.24 ± 0.13 d | 10.63 ± 0.17 b | 13.51 ± 0.11 a | 13.20 ± 0.31 a |
| C18:3 | 0.77 ± 0.00 a | 0.77 ± 0.00 a | 0.65 ± 0.02 b | 0.57 ± 0.02 c | 0.61 ± 0.04 b,c | 0.56 ± 0.06 c |
| C20:0 | 0.40 ± 0.00 b | 0.40 ± 0.01 b | 0.37 ± 0.00 b | 0.31 ± 0.09 b | 0.59 ± 0.07 a | 0.60 ± 0.06 a |
| C20:1 | 0.38 ± 0.02 c | 0.37 ± 0.02 c | 0.36 ± 0.03 c | 0.32 ± 0.02 c | 0.46 ± 0.04 b | 0.54 ± 0.03 a |
| C22:0 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.06 ± 0.03 a | 0.06 ± 0.03 a | 0.06 ± 0.02 a |
| C24:0 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.05 ± 0.02 a | 0.03 ± 0.01 b | 0.03 ± 0.01 b |
| SFA | 14.34 ± 0.27 b | 13.27 ± 0.03 c | 15.75 ± 0.65 a | 15.53 ± 0.30 a | 13.76 ± 0.04 b,c | 14.13 ± 0.75 b,c |
| MUFA | 74.52 ± 0.16 c | 77.15 ± 0.02 a | 75.36 ± 0.54 b | 73.27 ± 0.27 d | 72.12 ± 0.16 e | 72.10 ± 0.38 e |
| PUFA | 11.14 ± 0.11 b | 9.57 ± 0.01 c | 8.89 ± 0.11 d | 11.20 ± 0.15 b | 14.12 ± 0.15 a | 13.76 ± 0.37 a |
| O/L | 7.09 ± 0.06 c | 8.66 ± 0.01 b | 9.03 ± 0.08 a | 6.76 ± 0.12 d | 5.24 ± 0.06 e | 5.36 ± 0.09 e |
| Harvest Season | Pi * | Sampling | FFA (g 100 g−1) | PV (mEq O2 kg−1) | K232 | K270 |
|---|---|---|---|---|---|---|
| 1.92 | S2 | 0.61 ± 0.06 a | 7.49 ± 0.28 c | 1.94 ± 0.08 c | 0.21 ± 0.03 a,b | |
| 2017/2018 | 1.93 | S3 | 0.58 ± 0.07 a | 7.15 ± 0.23 c | 1.80 ± 0.12 d | 0.20 ± 0.06 a,b |
| 3.12 | S4 | 0.58 ± 0.06 a | 6.05 ± 0.28 d | 1.76 ± 0.02 d | 0.23 ± 0.04 a | |
| 2.43 | S2 | 0.52 ± 0.04 a,b | 8.92 ± 0.22 b | 2.34 ± 0.05 b | 0.17 ± 0.02 a,b | |
| 2019/2020 | 3.57 | S3 | 0.43 ± 0.06 b,c | 11.02 ± 0.48 a | 2.46 ± 0.10 a,b | 0.18 ± 0.03 a,b |
| 3.83 | S4 | 0.34 ± 0.07 c | 11.03 ± 0.67 a | 2.52 ± 0.04 a | 0.16 ± 0.03 b |
| Harvest Season | Pi * | Sampling | TPC | α-Tocopherol | β- and γ-Tocopherols | Total Tocopherols | Carotenoids | Chlorophylls |
|---|---|---|---|---|---|---|---|---|
| 1.92 | S2 | 761.64 ± 20.36 a | 246.20 ± 10.59 b | 5.28 ± 0.49 a,b | 251.47 ± 10.94 b | 57.77 ± 0.94 a | 54.68 ± 0.90 b | |
| 2017/2018 | 1.93 | S3 | 577.36 ± 15.33 b | 237.21 ± 4.49 b | 4.27 ± 0.82 b,c | 241.47 ± 4.82 b | 34.39 ± 0.30 c | 26.96 ± 0.10 d |
| 3.12 | S4 | 472.83 ± 12.60 c | 230.48 ± 14.03 b | 5.60 ± 0.73 a,b | 235.82 ± 13.45 b | 48.14 ± 0.95 b | 40.23 ± 0.73 c | |
| 2.43 | S2 | 592.86 ± 72.99 b | 349.74 ± 5.05 a | 6.32 ± 0.50 a | 356.06 ± 4.60 a | 47.27 ± 1.50 b | 66.30 ± 1.84 a | |
| 2019/2020 | 3.57 | S3 | 379.60 ± 8.43 d | 235.79 ± 34.25 b | 3.39 ± 1.44 c | 239.18 ± 35.67 b | 23.42 ± 1.71 d | 23.13 ± 0.44 f |
| 3.83 | S4 | 358.67 ± 25.97 d | 248.33 ± 11.82 b | 4.34 ± 0.17 b,c | 252.67 ± 11.99 b | 25.22 ± 0.91 d | 25.27 ± 0.52 e |
| Compound | S2 | S3 | S4 |
|---|---|---|---|
| 3,4-DHPEA | 0.33 ± 0.05 a,b | 0.42 ± 0.06 a | 0.25 ± 0.07 b |
| p-HPEA | 0.54 ± 0.03 b | 0.54 ± 0.02 b | 0.68 ± 0.05 a |
| Vanillic acid | 0.19 ± 0.02 a | 0.20 ± 0.02 a | 0.21 ± 0.06 a |
| Syringic acid | 0.44 ± 0.03 a | 0.29 ± 0.07 b | 0.36 ± 0.04 a,b |
| 3,4-DHPEA-EDA | 62.35 ± 3.29 a | 25.81 ± 4.57 b | 17.57 ± 1.77 c |
| 3,4-DHPEA-EDA-CARB | 1.64 ± 0.23 b | 5.65 ± 1.04 a | 1.31 ± 0.40 b |
| p-HPEA-EDA | 30.87 ± 0.93 a | 13.97 ± 0.78 c | 20.76 ± 1.40 b |
| Pinoresinol | 13.78 ± 0.28 a | 9.90 ± 0.69 c | 11.34 ± 0.51 b |
| Luteolin | 2.02 ± 0.23 b | 6.76 ± 0.62 a | 2.35 ± 2.15 b |
| p-HPEA-EA | 9.01 ± 0.94 a | 8.63 ± 1.68 a | 5.84 ± 1.27 b |
| Apigenin | 3.21 ± 0.33 a | 3.75 ± 1.27 a | 4.31 ± 1.50 a |
| Total | 124.38 ± 3.80 a | 75.92 ± 7.40 b | 64.98 ± 5.60 b |
| Compound | S2 | S3 | S4 |
|---|---|---|---|
| Methyl acetate | 2.28 ± 0.58 b | 1.82 ± 0.89 b | 5.25 ± 0.29 a |
| Ethyl acetate | 0.40 ± 0.05 b | 1.25 ± 0.49 a | 1.47 ± 0.03 a |
| 2-Methyl butanal | 1.83 ± 0.43 b | 1.65 ± 0.22 b | 5.18 ± 0.54 a |
| 3-Methyl butanal | 2.53 ± 0.57 b | 2.07 ± 0.18 b | 7.84 ± 0.44 a |
| 3-Pentanone | 1.25 ± 0.29 a | 1.64 ± 0.29 a | 1.69 ± 0.20 a |
| Pentanal | 1.07 ± 0.12 c | 13.78 ± 1.73 a | 4.00 ± 0.79 b |
| 3-Ethyl-1,5-octadiene | 15.81 ± 3.00 b | 37.05 ± 9.52 a | 16.60 ± 2.21 b |
| 1-Penten-3-one | 6.23 ± 0.67 c | 16.34 ± 0.84 a | 8.42 ± 0.78 b |
| Hexanal | 2.72 ± 0.44 b | 4.81 ± 0.42 a | 4.29 ± 0.85 a |
| (E)-2-Pentenal | 0.82 ± 0.19 a,b | 1.32 ± 0.24 a | 0.65 ± 0.32 b |
| 1-Penten-3-ol | 2.95 ± 0.34 c | 4.85 ± 0.42 b | 10.52 ± 0.15 a |
| (E)-2-Hexenal | 70.37 ± 9.18 b | 111.88 ± 14.16 a | 86.69 ± 7.13 a,b |
| (Z)-3-Hexen-1-yl acetate | 0.84 ± 0.20 b | 0.10 ± 0.03 b | 11.42 ± 1.11 a |
| (Z)-2-Penten-1-ol | 4.51 ± 0.46 c | 18.16 ± 1.30 a | 9.94 ± 1.07 b |
| Acetic acid | 3.69 ± 0.36 b | 8.48 ± 1.19 a | 3.59 ± 1.44 b |
| Hexan-1-ol | 1.49 ± 0.92 a | 1.10 ± 0.30 a | 1.99 ± 0.69 a |
| (Z)-3-Hexen-1-ol | 4.47 ± 0.31 a | 1.71 ± 0.09 c | 3.41 ± 0.44 b |
| Total | 123.25 ± 9.81 b | 228.00 ± 17.31 a | 182.95 ± 7.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squeo, G.; Silletti, R.; Mangini, G.; Summo, C.; Caponio, F. The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil. Foods 2021, 10, 369. https://doi.org/10.3390/foods10020369
Squeo G, Silletti R, Mangini G, Summo C, Caponio F. The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil. Foods. 2021; 10(2):369. https://doi.org/10.3390/foods10020369
Chicago/Turabian StyleSqueo, Giacomo, Roccangelo Silletti, Giacomo Mangini, Carmine Summo, and Francesco Caponio. 2021. "The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil" Foods 10, no. 2: 369. https://doi.org/10.3390/foods10020369
APA StyleSqueo, G., Silletti, R., Mangini, G., Summo, C., & Caponio, F. (2021). The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil. Foods, 10(2), 369. https://doi.org/10.3390/foods10020369