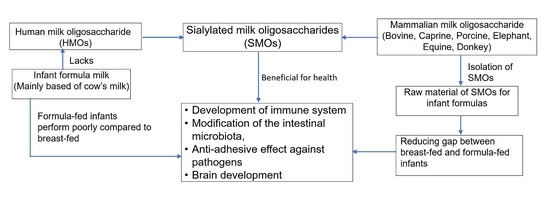
Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns
Abstract
:1. Introduction
1.1. Sialic Acid
1.2. Sialylated Milk Oligosaccharides in Human Breast Milk
2. Concentration and Distribution of SMOs in Other Animal Species
2.1. Bovine Milk
2.2. Caprine Milk
2.3. Porcine Milk
2.4. Elephantine Milk
2.5. Equine Milk
2.6. Donkey Milk
3. Sialylated Milk Oligosaccharides in Infant Formula Milk
4. Dose and Overall Functional Role of Sialylated Milk Oligosaccharides
5. Health Benefits of Sialylated Milk Oligosaccharides
5.1. Impact of SMOs on the Brain Development and Cognition
5.2. Impact of SMOs on the Gut Microbiota and Necrotising Enterocolitis (NEC)
6. Mechanisms via which Sialylated Milk Oligosaccharides Exerts Health Benefits
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newburg, D.S. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 2009, 87, 26–34. [Google Scholar] [CrossRef]
- Rønnestad, A.; Abrahamsen, T.G.; Medbø, S.; Reigstad, H.; Lossius, K.; Kaaresen, P.I.; Egeland, T.; Engelund, I.E.; Irgens, L.M.; Markestad, T. Late-onset septicemia in a Norwegian national cohort of extremely premature infants receiving very early full human milk feeding. Pediatrics 2005, 115, e269–e276. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Heinig, M.J.; Nommsen-Rivers, L.A. Differences in morbidity between breast-fed and formula-fed infants. J. Pediatr. 1995, 126, 696–702. [Google Scholar] [CrossRef]
- Beaudry, M.; Dufour, R.; Marcoux, S. Relation between infant feeding and infections during the first six months of life. J. Pediatr. 1995, 126, 191–197. [Google Scholar] [CrossRef]
- Duncan, B.; Ey, J.; Holberg, C.J.; Wright, A.L.; Martinez, F.D.; Taussig, L.M. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 1993, 91, 867–872. [Google Scholar] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An Exclusively Human Milk-Based Diet Is Associated with a Lower Rate of Necrotizing Enterocolitis than a Diet of Human Milk and Bovine Milk-Based Products. J. Pediatr. 2010, 156, 562–567.e561. [Google Scholar] [CrossRef] [Green Version]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized Trial of Exclusive Human Milk versus Preterm Formula Diets in Extremely Premature Infants. J. Pediatr. 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.L.; Michaelsen, K.F.; Sanders, S.A.; Reinisch, J.M. The Association between Duration of Breastfeeding and Adult Intelligence. JAMA 2002, 287, 2365–2371. [Google Scholar] [CrossRef] [Green Version]
- Uwaezuoke, S.N.; Eneh, C.I.; Ndu, I.K. Relationship Between Exclusive Breastfeeding and Lower Risk of Childhood Obesity: A Narrative Review of Published Evidence. Clin. Med. Insights Pediatr. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.F.; Alfenas, R.d.C.G.; Araújo, R.M.A. Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence. J. Pediatr. 2014, 90, 7–15. [Google Scholar] [CrossRef] [Green Version]
- WHO. Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 24 August 2020).
- ten Bruggencate, S.J.M.; Bovee-Oudenhoven, I.M.J.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H.C. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Mudd, A.T.; Fleming, S.A.; Labhart, B.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary sialyllactose influences sialic acid concentrations in the prefrontal cortex and magnetic resonance imaging measures in corpus callosum of young pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. In Personalized Nutrition for the Diverse Needs of Infants and Children; Bier, D.M., German, J.B., Lönnerdal, B., Eds.; Karger Publishers: Basel, Switzerland, 2008; Volume 62, pp. 205–222. [Google Scholar]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk. The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-Milk Glycans That Inhibit Pathogen Binding Protect Breast-feeding Infants against Infectious Diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef] [Green Version]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci. Glycotechnol. 2018, 30, SE51–SE65. [Google Scholar] [CrossRef] [Green Version]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [Green Version]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Coppa, G.V.; Bruni, S.; Morelli, L.; Soldi, S.; Gabrielli, O. The first prebiotics in humans: Human milk oligosaccharides. J. Clin. Gastroenterol. 2004, 38, S80–S83. [Google Scholar] [CrossRef]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Han, N.S.; Kim, T.-J.; Park, Y.-C.; Kim, J.; Seo, J.-H. Biotechnological production of human milk oligosaccharides. Biotechnol. Adv. 2012, 30, 1268–1278. [Google Scholar] [CrossRef]
- Wang, H.X.; Chen, Y.; Haque, Z.; de Veer, M.; Egan, G.; Wang, B. Sialylated milk oligosaccharides alter neurotransmitters and brain metabolites in piglets: An In vivo magnetic resonance spectroscopic (MRS) study. Nutr. Neurosci. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.D.; Zandberg, W.F. Quantitation of sialic acids in infant formulas by liquid chromatography–mass spectrometry: An assessment of different protein sources and discovery of new analogues. J. Agric. Food Chem. 2018, 66, 8114–8123. [Google Scholar] [CrossRef]
- Claumarchirant, L.; Sanchez-Siles, L.M.; Matencio, E.; Alegría, A.; Lagarda, M.J. Evaluation of sialic acid in infant feeding: Contents and bioavailability. J. Agric. Food Chem. 2016, 64, 8333–8342. [Google Scholar] [CrossRef]
- Bulai, T.; Bratosin, D.; Pons, A.; Montreuil, J.; Zanetta, J.P. Diversity of the human erythrocyte membrane sialic acids in relation with blood groups. FEBS Lett. 2003, 534, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Robbe, C.; Capon, C.; Maes, E.; Rousset, M.; Zweibaum, A.; Zanetta, J.-P.; Michalski, J.-C. Evidence of regio-specific glycosylation in human intestinal mucins presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 2003, 278, 46337–46348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Karim, M.; Wang, B. Is sialic acid in milk food for the brain. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 18–29. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef]
- Martín-Sosa, S.; Martín, M.-J.; García-Pardo, L.A.; Hueso, P. Distribution of sialic acids in the milk of Spanish mothers of full term infants during lactation. J. Pediatric Gastroenterol. Nutr. 2004, 39, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Kooner, A.S.; Yu, H.; Chen, X. Synthesis of N-Glycolylneuraminic Acid (Neu5Gc) and Its Glycosides. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Ji, S.; Wang, F.; Chen, Y.; Yang, C.; Zhang, P.; Zhang, X.; Troy, F.A.; Wang, B. Developmental changes in the level of free and conjugated sialic acids, Neu5Ac, Neu5Gc and KDN in different organs of pig: A LC-MS/MS quantitative analyses. Glycoconj. J. 2017, 34, 21–30. [Google Scholar] [CrossRef]
- Brunngraber, E.G.; Witting, L.A.; Haberland, C.; Brown, B. Glycoproteins in Tay-sachs disease: Isolation and carbohydrate composition of glycopeptides. Brain Res. 1972, 38, 151–162. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.C.; Du, Y. Characterization of porcine milk oligosaccharides over lactation between primiparous and multiparous female pigs. Sci. Rep. 2018, 8, 4688. [Google Scholar] [CrossRef]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human Milk Oligosaccharides Inhibit the Adhesion to Caco-2 Cells of Diarrheal Pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Tao, N.; DePeters, E.J.; Freeman, S.; German, J.B.; Grimm, R.; Lebrilla, C.B. Bovine Milk Glycome. J. Dairy Sci. 2008, 91, 3768–3778. [Google Scholar] [CrossRef] [Green Version]
- Aldredge, D.L.; Geronimo, M.R.; Hua, S.; Nwosu, C.C.; Lebrilla, C.B.; Barile, D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology 2013, 23, 664–676. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Barile, D.; Meyrand, M.; Poulsen, N.A.; Larsen, L.B.; Lebrilla, C.B.; German, J.B.; Bertram, H.C. Natural variability in bovine milk oligosaccharides from Danish Jersey and Holstein-Friesian breeds. J. Agric. Food Chem. 2012, 60, 6188–6196. [Google Scholar] [CrossRef]
- Robinson, R.C.; Poulsen, N.A.; Colet, E.; Duchene, C.; Larsen, L.B.; Barile, D. Profiling of aminoxyTMT-labeled bovine milk oligosaccharides reveals substantial variation in oligosaccharide abundance between dairy cattle breeds. Sci. Rep. 2019, 9, 5465. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Lootah, M.; Tahlak, M.; Venema, K. Profiles of Human Milk Oligosaccharides and Their Relations to the Milk Microbiota of Breastfeeding Mothers in Dubai. Nutrients 2020, 12, 1727. [Google Scholar] [CrossRef]
- Bao, Y.; Newburg, D.S. Capillary electrophoresis of acidic oligosaccharides from human milk. Electrophoresis 2008, 29, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ding, J.; Liang, X. Chromatographic methods for the analysis of oligosaccharides in human milk. Anal. Methods 2017, 9, 1071–1077. [Google Scholar] [CrossRef]
- Veh, R.W.; Michalski, J.-C.; Corfield, A.P.; Sander-Wewer, M.; Gies, D.; Schauer, R. New chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides. J. Chromatogr. A 1981, 212, 313–322. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawase, H.; Kimura, K.; Watanabe, Y.; Ohtani, M.; Arai, I.; Urashima, T. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J. Dairy Sci. 2003, 86, 1315–1320. [Google Scholar] [CrossRef] [Green Version]
- McJarrow, P.; van Amelsfort-Schoonbeek, J. Bovine sialyl oligosaccharides: Seasonal variations in their concentrations in milk, and a comparison of the colostrums of Jersey and Friesian cows. Int. Dairy J. 2004, 14, 571–579. [Google Scholar] [CrossRef]
- Claps, S.; Di Napoli, M.A.; Caputo, A.R.; Rufrano, D.; Sepe, L.; Di Trana, A. Factor affecting the 3′ sialyllactose, 6′ sialyllactose and disialyllactose content in caprine colostrum and milk: Breed and parity. Small Rumin. Res. 2016, 134, 8–13. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine milk oligosaccharides and sialic acid concentrations vary throughout lactation. Front. Nutr. 2016, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Kunz, C.; Rudloff, S.; Schad, W.; Braun, D. Lactose-derived oligosaccharides in the milk of elephants: Comparison with human milk. Br. J. Nutr. 1999, 82, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Difilippo, E.; Willems, H.A.M.; Vendrig, J.C.; Fink-Gremmels, J.; Gruppen, H.; Schols, H.A. Comparison of Milk Oligosaccharides Pattern in Colostrum of Different Horse Breeds. J. Agric. Food Chem. 2015, 63, 4805–4814. [Google Scholar] [CrossRef]
- Monti, L.; Cattaneo, T.M.P.; Orlandi, M.; Curadi, M.C. Capillary electrophoresis of sialylated oligosaccharides in milk from different species. J. Chromatogr. A 2015, 1409, 288–291. [Google Scholar] [CrossRef]
- Licitra, R.; Li, J.; Liang, X.; Altomonte, I.; Salari, F.; Yan, J.; Martini, M. Profile and content of sialylated oligosaccharides in donkey milk at early lactation. LWT 2019, 115, 108437. [Google Scholar] [CrossRef]
- Tomotake, H.; Okuyama, R.; Katagiri, M.; Fuzita, M.; Yamato, M.; Ota, F. Comparison between Holstein Cow’s Milk and Japanese-Saanen Goat’s Milk in Fatty Acid Composition, Lipid Digestibility and Protein Profile. Biosci. Biotechnol. Biochem. 2006, 70, 2771–2774. [Google Scholar] [CrossRef]
- Kim, H.-H.; Yun, S.-S.; Oh, C.-H.; Yoon, S.-S. Galactooligosaccharide and Sialyllactose Content in Commercial Lactose Powders from Goat and Cow Milk. Korean J. Food Sci. Anim. Resour. 2015, 35, 572–576. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, S.S.; Te Poele, E.M.; Chatziioannou, A.C.; Benjamins, E.; Haandrikman, A.; Dijkhuizen, L. Goat Milk Oligosaccharides: Their Diversity, Quantity, and Functional Properties in Comparison to Human Milk Oligosaccharides. J. Agric. Food Chem. 2020, 68, 13469–13485. [Google Scholar] [CrossRef]
- Martín-Ortiz, A.; Salcedo, J.; Barile, D.; Bunyatratchata, A.; Moreno, F.J.; Martin-García, I.; Clemente, A.; Sanz, M.L.; Ruiz-Matute, A.I. Characterization of goat colostrum oligosaccharides by nano-liquid chromatography on chip quadrupole time-of-flight mass spectrometry and hydrophilic interaction liquid chromatography-quadrupole mass spectrometry. J. Chromatogr. A 2016, 1428, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Zhang, Y.; Song, B.; Zhang, S.; Pang, X.; Sari, R.N.; Liu, L.; Wang, J.; Lv, J. Comparative analysis of oligosaccharides in Guanzhong and Saanen goat milk by using LC–MS/MS. Carbohydr. Polym. 2020, 235, 115965. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, Y.R.F.; da Silva Vasconcelos, M.A.; Costa, R.G.; de Azevedo Filho, C.A.; de Paiva, E.P.; Queiroga, R.d.C.R.d.E. Sialic acid content of goat milk during lactation. Livest. Sci. 2015, 177, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, J.; Frese, S.A.; Mills, D.A.; Barile, D. Characterization of porcine milk oligosaccharides during early lactation and their relation to the fecal microbiome. J. Dairy Sci. 2016, 99, 7733–7743. [Google Scholar] [CrossRef] [Green Version]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. Milk oligosaccharide variation in sow milk and milk oligosaccharide fermentation in piglet intestine. J. Agric. Food Chem. 2016, 64, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, M.; Wynn, P.; Wang, B. Molecular characterization of the level of sialic acids N-acetylneuraminic acid, N-glycolylneuraminic acid, and ketodeoxynonulosonic acid in porcine milk during lactation. J. Dairy Sci. 2016, 99, 8431–8442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, B.L.; Hart, L.A.; Pinter-Wollman, N. Large brains and cognition: Where do elephants fit in? Neurosci. Biobehav. Rev. 2008, 32, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Salcedo, J.; Frese, S.A.; Barile, D. Thoroughbred mare’s milk exhibits a unique and diverse free oligosaccharide profile. FEBS Open Bio 2018, 8, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Lane, J.A.; Marino, K.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lei, B.; Yan, J.; Li, J.; Zhou, X.; Ren, F.; Guo, H. Donkey milk oligosaccharides influence the growth-related characteristics of intestinal cells and induce G2/M growth arrest via the p38 pathway in HT-29 cells. Food Funct. 2019, 10, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ling, P.-R.; Blackburn, G. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef]
- Martin-Sosa, S.; Martín, M.-J.; García-Pardo, L.-A.; Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: Variations with the progression of lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Vacca-Smith, A.; Van Wuyckhuyse, B.; Tabak, L.; Bowen, W. The effect of milk and casein proteins on the adherence of Streptococcus mutans to saliva-coated hydroxyapatite. Arch. Oral Biol. 1994, 39, 1063–1069. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Benno, Y.; Sawada, K.; Mitsuoka, T. The intestinal microflora of infants: Composition of fecal flora in breast-fed and bottle-fed infants. Microbiol. Immunol. 1984, 28, 975–986. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, A. The Chemistry and Biology of Sialic Acids and Related Substances; Cambridge University Press: Cambridge, UK, 1960. [Google Scholar]
- Schauer, R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1981, 40, 131–234. [Google Scholar]
- Chou, H.-H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Satta, Y.; Gagneux, P.; Varki, A.; Takahata, N. Alu-mediated inactivation of the human CMP-N-acetylneuraminic acid hydroxylase gene. Proc. Natl. Acad. Sci. USA 2001, 98, 11399–11404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samraj, A.N.; Pearce, O.M.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, M.; Thomson, P.C.; Wynn, P.C.; Wang, B. The non-human glycan, N-glycolylneuraminic acid (Neu5Gc), is not expressed in all organs and skeletal muscles of nine animal species. Food Chem. 2020, 128439. [Google Scholar] [CrossRef]
- Hurum, D.; Rohrer, J. Determination of sialic acids in infant formula by chromatographic methods: A comparison of high-performance anion-exchange chromatography with pulsed amperometric detection and ultra-high-performance liquid chromatography methods. J. Dairy Sci. 2012, 95, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.H.; Kim, D.H.; Gurung, R.B.; Donovan, S.M. Evaluation of 6′-Sialyllactose Sodium Salt Supplementation to Formula on Growth and Clinical Parameters in Neonatal Piglets. Nutrients 2020, 12, 1030. [Google Scholar] [CrossRef] [Green Version]
- Phipps, K.R.; Baldwin, N.J.; Lynch, B.; Stannard, D.R.; Šoltésová, A.; Gilby, B.; Mikš, M.H.; Röhrig, C.H. Toxicological safety assessment of the human-identical milk oligosaccharide 3′-sialyllactose sodium salt. J. Appl. Toxicol. 2019, 39, 1378–1393. [Google Scholar] [CrossRef]
- Kawashima, N.; Yoon, S.-J.; Itoh, K.; Nakayama, K.-i. Tyrosine Kinase Activity of Epidermal Growth Factor Receptor Is Regulated by GM3 Binding through Carbohydrate to Carbohydrate Interactions. J. Biol. Chem. 2009, 284, 6147–6155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowardin, C.A.; Ahern, P.P.; Kung, V.L.; Hibberd, M.C.; Cheng, J.; Guruge, J.L.; Sundaresan, V.; Head, R.D.; Barile, D.; Mills, D.A.; et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc. Natl. Acad. Sci. USA 2019, 116, 11988–11996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obelitz-Ryom, K.; Bering, S.B.; Overgaard, S.H.; Eskildsen, S.F.; Ringgaard, S.; Olesen, J.L.; Skovgaard, K.; Pankratova, S.; Wang, B.; Brunse, A. Bovine Milk Oligosaccharides with Sialyllactose Improves Cognition in Preterm Pigs. Nutrients 2019, 11, 1335. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, P.; Fang, W.; Chen, Y.; Zhang, N.; Qiao, Z.; Troy, F.A.; Wang, B. Molecular Mechanisms Underlying How Sialyllactose Intervention Promotes Intestinal Maturity by Upregulating GDNF Through a CREB-Dependent Pathway in Neonatal Piglets. Mol. Neurobiol. 2019, 56, 7994–8007. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R. The human milk oligosaccharides 2′-fucosyllactose and 6′-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Fuhrer, A.; Sprenger, N.; Kurakevich, E.; Borsig, L.; Chassard, C.; Hennet, T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J. Exp. Med. 2010, 207, 2843–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.-W.; Kim, E.-Y.; Kim, S.-J.; Choi, H.-J.; Jang, S.B.; Kim, K.-J.; Ha, S.-H.; Abekura, F.; Kwak, C.-H.; Kim, C.-H.; et al. Sialyllactose suppresses angiogenesis by inhibiting VEGFR-2 activation, and tumor progression. Oncotarget 2017, 8, 58152–58162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.S.; Joo, W.; Ling, L.; Choi, H.S.; Han, N.S. In vitro digestion and fermentation of sialyllactoses by infant gut microflora. J. Funct. Foods 2016, 21, 497–506. [Google Scholar] [CrossRef]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern. Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kang, L.-J.; Lee, K.M.; Cho, C.; Song, E.K.; Kim, W.; Park, T.J.; Yang, S. 3′-Sialyllactose protects against osteoarthritic development by facilitating cartilage homeostasis. J. Cell. Mol. Med. 2018, 22, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Marotta, M.; Ryan, J.T.; Hickey, R.M. The predominant milk oligosaccharide 6′-sialyllactose reduces the internalisation of Pseudomonas aeruginosa in human pneumocytes. J. Funct. Foods 2014, 6, 367–373. [Google Scholar] [CrossRef]
- Yang, L.; Emerich, H. 3′ Sialyllactose Inhibits Adhesion of Diarrheagenic Bacteria to Epithelial Monolayers in vitro. Faseb J. 2013, 27, 611–629. [Google Scholar] [CrossRef]
- Ha, S.-H.; Kwak, C.-H.; Park, J.-Y.; Abekura, F.; Lee, Y.-C.; Kim, J.-s.; Chung, T.-W.; Kim, C.-H. 3′-sialyllactose targets cell surface protein, SIGLEC-3, and induces megakaryocyte differentiation and apoptosis by lipid raft-dependent endocytosis. Glycoconj. J. 2020, 1–14. [Google Scholar] [CrossRef]
- Perdijk, O.; Van Baarlen, P.; Fernandez-Gutierrez, M.M.; Van Den Brink, E.; Schuren, F.H.; Brugman, S.; Savelkoul, H.F.; Kleerebezem, M.; Van Neerven, R. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.J.; Kwon, E.S.; Lee, K.M.; Cho, C.; Lee, J.I.; Ryu, Y.B.; Youm, T.H.; Jeon, J.; Cho, M.R.; Jeong, S.Y. 3′-Sialyllactose as an inhibitor of p65 phosphorylation ameliorates the progression of experimental rheumatoid arthritis. Br. J. Pharmacol. 2018, 175, 4295–4309. [Google Scholar] [CrossRef] [Green Version]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacs, E.; Fischl, B.; Quinn, B.; Chong, W.; Gadian, D.; Lucas, A. Impact of breast milk on IQ, brain size and white matter development. Pediatric Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Krol, K.M.; Grossmann, T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt-Gesundh. Gesundh. 2018, 61, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laventhal, N.; Tarini, B.A.; Lantos, J. Ethical issues in neonatal and pediatric clinical trials. Pediatr. Clin. 2012, 59, 1205–1220. [Google Scholar] [CrossRef] [Green Version]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.A. Long-chain polyunsaturated fatty acids and infant development. Lancet 1999, 354, 1919–1920. [Google Scholar] [CrossRef]
- Balehegn, M.; Mekuriaw, Z.; Miller, L.; Mckune, S.; Adesogan, A.T. Animal-sourced foods for improved cognitive development. Anim. Front. 2019, 9, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprenger, N.; Duncan, P.I. Sialic acid utilization. Adv. Nutr. 2012, 3, 392S–397S. [Google Scholar] [CrossRef] [Green Version]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Downing, J.A.; Petocz, P.; Brand-Miller, J.; Bryden, W.L. Metabolic fate of intravenously administered N-acetylneuraminic acid-6-14C in newborn piglets. Asia Pac. J. Clin. Nutr. 2007, 16, 110–115. [Google Scholar] [PubMed]
- Nohle, U.; Schauer, R. Uptake, metabolism and excretion of orally and intravenously administered, 14C-and 3H-labeled N-acetylneuraminic acid mixture in the mouse and rat. Biol. Chem. 1981, 362, 1495–1506. [Google Scholar]
- Heine, W.; Wutzke, K.D.; Radke, M. Sialic acid in breast milk and infant formula food. Mon. Kinderheilkd. 1993, 141, 946–950. [Google Scholar]
- Keenan, T.; Huang, C.; Morré, D.J. Gangliosides: Nonspecific localization in the surface membranes of bovine mammary gland and rat liver. Biochem. Biophys. Res. Commun. 1972, 47, 1277–1283. [Google Scholar] [CrossRef]
- Becker, C.G.; Artola, A.; Gerardy-Schahn, R.; Becker, T.; Welzl, H.; Schachner, M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 1996, 45, 143–152. [Google Scholar] [CrossRef]
- Rahmann, H. Brain gangliosides and memory formation. Behav. Brain Res. 1995, 66, 105–116. [Google Scholar] [CrossRef]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.; Buck, R.; Rueda, R.; Martín, M. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef] [Green Version]
- Sakai, F.; Ikeuchi, Y.; Urashima, T.; Fujihara, M.; Ohtsuki, K.; Yanahira, S. Effects of feeding sialyllactose and galactosylated N-acetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. J. Appl. Glycosci. 2006, 53, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Bertelsen, R.; Jensen, E.; Ringel-Kulka, T. Use of probiotics and prebiotics in infant feeding. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Egert, M.; Bassett, S.A.; Bibiloni, R. Detection of sialic acid-utilising bacteria in a caecal community batch culture using RNA-based stable isotope probing. Nutrients 2015, 7, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Idota, T.; Kawakami, H.; Nakajima, I. Growth-promoting effects of N-acetylneuraminic acid-containing substances on bifidobacteria. Biosci. Biotechnol. Biochem. 1994, 58, 1720–1722. [Google Scholar] [CrossRef]
- Kavanaugh, D.W.; O’Callaghan, J.; Butto, L.F.; Slattery, H.; Lane, J.; Clyne, M.; Kane, M.; Joshi, L.; Hickey, R.M. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS ONE 2013, 8, e67224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Sosa, S.; Martín, M.-J.; Hueso, P. The Sialylated Fraction of Milk Oligosaccharides Is Partially Responsible for Binding to Enterotoxigenic and Uropathogenic Escherichia coli Human Strains. J. Nutr. 2002, 132, 3067–3072. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-J.; Kim, J.W. Bacterial clearance is enhanced by α2, 3-and α2, 6-sialyllactose via receptor-mediated endocytosis and phagocytosis. Infect. Immun. 2019, 87, e00618–e00694. [Google Scholar] [PubMed] [Green Version]
- Autran, C.A.; Schoterman, M.H.; Jantscher-Krenn, E.; Kamerling, J.P.; Bode, L. Sialylated galacto-oligosaccharides and 2′-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br. J. Nutr. 2016, 116, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Mahmood, S.; Nagpaul, J.P.; Mahmood, A. Functional role of sialic acid in IgG binding to microvillus membranes in neonatal rat intestine. Neonatology 1999, 76, 55–64. [Google Scholar] [CrossRef]
- Dickson, J.J.; Messer, M. Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to the sialic acid content of milk. Biochem. J. 1978, 170, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Colostrum (g/L) | Mature Milk (g/L) | References | |||
|---|---|---|---|---|---|
| 3′-SL | 6′-SL | 3′-SL | 6′-SL | ||
| Human Milk | 0.09–0.35 | 0.25–1.30 | 0.17–0.50 | 0.17–0.50 | [12,54,55,56] |
| Bovine Milk | 0.09–1.25 | 0.03–0.24 | 0.04–0.12 | 0.01–0.09 | [12,57,58,59] |
| Caprine Milk | 0.18–0.25 | 0.10–0.13 | 0.03–0.09 | 0.05–0.07 | [60,61] |
| Porcine Milk | 0.09 | N/A | 0.01 | N/A | [62] |
| Elephant Milk | 1.89 (d45) | 0.30 (d45) | 0.86 | 0.34 | [63] |
| Mare Milk | 0.29–1.75 | 0.02–0.23 | 0.06–0.08 | 0.0004–0.0026 | [64,65] |
| Donkey Milk | 0.02 (d15) | 0.02 (d15) | 0.01–0.02 | 0.01–0.02 | [65,66] |
| Study Type | Breast-Fed Infants | Formula-Feeding Infants | References |
|---|---|---|---|
| 30 breast-fed vs. 60 formula-fed (30 for formula A and 30 for formula B) babies recruited before or right after birth and exclusive breast/formula feeding for more than 4 months | At 40 days old ↓ diversity ↑ Bifidobacterium ↑ Bacteroides ↓ Lachnospiracea ↓ Streptococcus ↓ Enterococcus ↓ Veillonella ↓ Clostridioid At 3 months old ↓ Lachnospiracea ↓ Clostridioid | At 40 days old ↑ diversity ↓ Bifidobacterium ↓ Bacteroides ↑ Lachnospiracea ↑ Streptococcus ↑ Enterococcus ↑ Veillonella ↑ Clostridioid At 3 months old ↑ Lachnospiracea ↑ Clostridioid | [85] |
| 6 breast-fed (11–22 days old) vs. 6 formula-fed (14 to 36 days old) | ↑ Bifidobacterium | ↑ microbial diversity ↑ Atopobium ↓ Bifidobacterium ↑ Bacteroides | [86] |
| 700 breast-fed infants vs. 232 formula-fed infants at 1 month of age | ↑ Bifidobacteria | ↑ E.coli ↑ C.difficile ↑ Bacteroides ↑ Lactobacilli | [87] |
| 35 breast-fed vs. 35 formula-fed (28 to 46 days old) | ↑ Clostridium paraputrificum ↑ C.perfringens ↑ Bacillus subtilis ↑ C. clostridifforme ↑ Bacteroides vulgatus ↑ Veillonella parvula ↑ Lactobacillus acidophilus ↑ E. coli ↑ Streptococcus bovis ↑ S. faecalis ↑ S. faecium ↑ C. difficile ↑ C. tertium ↑ Pseudomonas aeruginosa | [88] | |
| 16 breast-fed vs. 6 formula-fed infants at 3 months | ↑ Bacteroides ↓ Clostridium XVIII ↓ Lachnospiracea incerate sedis ↓ Enterococcus ↓ Veillonella | [89] |
| Species | Type of SMO | Effect | References |
|---|---|---|---|
| In vivo studies | |||
| Piglet | 3′SL and 6′SL |
| [102] |
| Piglet | 3′SL and 6′SL |
| [103] |
| Piglet | 3′SL and 6′SL |
| [30] |
| Piglet | 3′SL and 6′SL |
| [16] |
| Premature Ppiglets, newborn mice, and human intestinal explants with NEC | 3′SL |
| [104] |
| Germ-Free mice colonized with microbiome of stunted 6-month- old | Bovine SMOs, mainly SL |
| [101] |
| Mice | SL-deficient mother |
| [105] |
| Mice | 3′SL or 6′SL |
| [106] |
| In vivo mouse models of Lewis lung carcinoma, melanoma, and colon carcinoma cells | 3′SL |
| [107] |
| In vitro studies | |||
| In vitro microplate study | 3′SL and 6′SL |
| [108] |
| Human colon carcinoma Caco-2 cell lines | 6′SL |
| [109] |
| In vitro and ex vivo experiments using mouse model | 3′SL |
| [110] |
| In vitro invasion assay using Adherent A549 cells | 6′SL and 3′SL |
| [111] |
| In vitro study using epithelial monolayers | 3′SL |
| [112] |
| In vitro study using human chronic myeloid leukemia cell line K562 | 3′SL |
| [113] |
| Adult and infant human epithelial cell lines and fecal batch cultures | 3′SL and 6′SL |
| [114] |
| In vitro and in vivo mouse model of collagen-induced arthritis | 3′SL |
| [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods 2021, 10, 473. https://doi.org/10.3390/foods10020473
Hobbs M, Jahan M, Ghorashi SA, Wang B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods. 2021; 10(2):473. https://doi.org/10.3390/foods10020473
Chicago/Turabian StyleHobbs, Madalyn, Marefa Jahan, Seyed A. Ghorashi, and Bing Wang. 2021. "Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns" Foods 10, no. 2: 473. https://doi.org/10.3390/foods10020473
APA StyleHobbs, M., Jahan, M., Ghorashi, S. A., & Wang, B. (2021). Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods, 10(2), 473. https://doi.org/10.3390/foods10020473