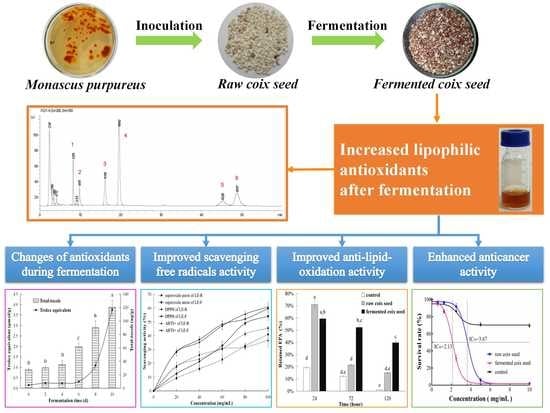
Increases of Lipophilic Antioxidants and Anticancer Activity of Coix Seed Fermented by Monascus purpureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fermentation of Coix Seed Using M. purpureus Strain
2.3. Extraction and Determination of Lipophilic Antioxidants and Coixenolide in Raw and Fermented Coix Seed
2.4. Determination of Free-Radical-Scavenging Activity of the Lipophilic Extract
2.5. Determination of Anti-Lipid-Oxidation Activity Using Fatty Acid Model
2.6. Determination of Anticancer Activity of Lipophilic Extract on HEp2 Cells
2.7. Data Analysis
3. Results and Discussion
3.1. Lipophilic Antioxidants and Coixenolide in Raw and Fermented Coix Seed
3.2. Scavenging Activities on Different Free Radicals of Lipophilic Extracts from Raw and Fermented Coix Seed
3.3. Anti-Lipid-Oxidation Activities of Lipophilic Extracts from Raw and Fermented Coix Seeds
3.4. Anticancer Activities of Lipophilic Extracts from Raw and Fermented Coix Seeds
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tseng, Y.; Yang, J.; Chang, H.; Mau, J. Taste quality of monascal adlay. J. Agric. Food Chem. 2004, 52, 2297–2300. [Google Scholar] [CrossRef]
- Zhu, F. Coix: Chemical composition and health effects. Trends Food Sci. Technol. 2017, 61, 160–175. [Google Scholar] [CrossRef]
- Huang, D.; Kuo, Y.; Lin, F.; Lin, Y.; Chiang, W. Effect of Adlay (Coix lachryma-jobi L. var. Ma-yuen Stapf) Testa and its phenolic components on Cu2+-treated Low-Density Lipoprotein (LDL) oxidation and Lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. J. Agric. Food Chem. 2009, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Qin, L.; Zeng, H.; Zhu, Y. Comprehensive evaluation of physicochemical properties and antioxidant activity of B. Subtili-fermented polished adlay subjected to different drying methods. Food Sci. Nutr. 2020, 8, 2124–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Chung, C.; Chiang, W.; Lin, Y. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef]
- Xi, X.; Zhu, Y.; Tong, Y.; Yang, X.; Tang, N.; Ma, S.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different Job’s tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e153269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Howard, L.R. Analysis Methods of Phenolic Acids; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, H.; Zeng, H.; Prinyawiwatukul, W.; Xu, W.; Xu, Z. Increases in phenolic, fatty acid, and phytosterol contents and anticancer activities of sweet potato after fermentation by Lactobacillus acidophilus. J. Agric. Food Chem. 2018, 66, 2735–2741. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Zeng, J.; Tian, X.; Bei, Q.; Wu, Z. Enhancing three phenolic fractions of oats (Avena sativa L.) and their antioxidant activities by solid-state fermentation with Monascus anka and Bacillus subtilis. J. Cereal Sci. 2020, 93, 102940. [Google Scholar] [CrossRef]
- Marič, A.; Skočaj, M.; Likar, M.; Sepčić, K.; Cigić, I.K.; Grundner, M.; Gregori, A. Comparison of lovastatin, citrinin and pigment production of different Monascus purpureus strains grown on rice and millet. J. Food Sci. Technol. 2019, 56, 3364–3373. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.; da Silva Fernandes, M.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Shen, Y.; Du, L.; Zeng, H.; Zhang, X.; Prinyawiwatkul, W.; Alonso Marenco, J.R.; Xu, Z. Butterfly pea (Clitoria ternatea) seed and petal extracts decreased HEp-2 carcinoma cell viability. Int. J. Food Sci. Technol. 2016, 51, 1860–1868. [Google Scholar] [CrossRef]
- Jang, S.; Xu, Z. Lipophilic and hydrophilic antioxidants and their antioxidant activities in purple rice bran. J. Agric. Food Chem. 2009, 57, 858–862. [Google Scholar] [CrossRef]
- Yang, J.; Tseng, Y.; Chang, H.; Lee, Y.; Mau, J. Storage stability of monascal adlay. Food Chem. 2005, 90, 303–309. [Google Scholar] [CrossRef]
- Du, L.; Shen, Y.; Zhang, X.; Prinyawiwatkul, W.; Xu, Z. Antioxidant-rich phytochemicals in miracle berry (Synsepalum dulcificum) and antioxidant activity of its extracts. Food Chem. 2014, 153, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Fan, S.T.; Huang, D.F.; Yu, Q.; Liu, X.Z.; Li, C.; Wang, S.; Xiong, T.; Nie, S.P.; Xie, M.Y. Effect of Lactobacillus plantarum NCU116 fermentation on Asparagus officinalis polysaccharide: Characterization, antioxidative, and immunoregulatory activities. J. Agric. Food Chem. 2018, 66, 10703–10711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, Y.; Prinyawiwatkul, W.; King, J.M.; Xu, Z. Comparison of the activities of hydrophilic anthocyanins and lipophilic tocols in black rice bran against lipid oxidation. Food Chem. 2013, 141, 111–116. [Google Scholar] [CrossRef]
- Chen, G.; Yang, S.; Wang, C.; Shi, K.; Zhao, X.; Wu, Z. Investigation of the mycelial morphology of Monascus and the expression of pigment biosynthetic genes in high-salt-stress fermentation. Appl. Microbiol. Biotechnol. 2020, 104, 2469–2479. [Google Scholar] [CrossRef]
- Masiero, S.S.; Peretti, A.; Trierweiler, L.F.; Trierweiler, J.O. Simultaneous cold hydrolysis and fermentation of fresh sweet potato. Biomass Bioenergy 2014, 70, 174–183. [Google Scholar] [CrossRef]
- Moreau, R.A.; Lampi, A.M. Analysis Methods for Tocopherols and Tocotrienols; Xu, Z., Howard, L.R., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 353–386. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Yew, W.W.; Wahid, M.B. Tocotrienols and cancer: Beyond antioxidant activity. Eur. J. Lipid Sci. Technol. 2007, 109, 445–452. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Nyström, L. Analysis Methods of Phytosterols; Xu, Z., Howard, L.R., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 313–351. [Google Scholar] [CrossRef]
- Lesma, G.; Luraghi, A.; Bavaro, T.; Bortolozzi, R.; Rainoldi, G.; Roda, G.; Viola, G.; Ubiali, D.; Silvani, A. Phytosterol and γ-Oryzanol conjugates: Synthesis and evaluation of their antioxidant, antiproliferative, and anticholesterol activities. J. Nat. Prod. 2018, 81, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, R.J.; Xu, Z.; Godber, J.S.; Lanfer-Marquez, U.M. Tocopherols, tocotrienols, and γ-oryzanol contents in Japonica and Indica subspecies of rice (Oryza sativa L.) cultivated in Brazil. Cereal Chem. 2008, 85, 243–247. [Google Scholar] [CrossRef]
- Massarolo, K.C.; de Souza, T.D.; Collazzo, C.C.; Furlong, E.B.; Souza Soares, L.A. The impact of Rhizopus oryzae cultivation on rice bran: Gamma-oryzanol recovery and its antioxidant properties. Food Chem. 2017, 228, 43–49. [Google Scholar] [CrossRef]
- Cho, J.; Lee, H.J.; Kim, G.A.; Kim, G.D.; Lee, Y.S.; Shin, S.C.; Park, K.; Moon, J. Quantitative analyses of individual γ-oryzanol (steryl ferulates) in conventional and organic brown rice (Oryza sativa L.). J. Cereal Sci. 2012, 55, 337–343. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Zhu, Y.; Xu, Z. Assessment of the correlations between reducing power, DPPH scavenging activity and anti-lipid-oxidation capability of phenolic antioxidants. LWT Food Sci. Technol. 2015, 63, 569–574. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Yi, B.; Lee, J.; Kim, M. Increasing oxidative stability in corn oils through extraction of γ-oryzanol from heat treated rice bran. J. Cereal Sci. 2020, 91, 102880. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, R.; Cao, X.; Liu, X.; Qin, X. Improved physicochemical properties of yogurt fortified with fish oil/γ-oryzanol by nanoemulsion technology. Molecules 2018, 23, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, R.; Ramroth, H.; Becher, H.; Dietz, A.; Schmezer, P.; Popanda, O. Laryngeal cancer risk associated with smoking and alcohol consumption is modified by genetic polymorphisms in ERCC5, ERCC6 and RAD23B but not by polymorphisms in five other nucleotide excision repair genes. Int. J. Cancer 2009, 125, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, R.; Gupta, S.C.; Ji, H.K.; Aggarwal, B.B. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2011, 7, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S. Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef]







| Compound | Raw Coix Seed | Fermented Coix Seed |
|---|---|---|
| α-Tocopherol (μg/g DW) | 0.1 ± 0.1 A | 17.9 ± 7.1 B |
| α-Tocotrienol (μg/g DW) | 2.4 ± 0.6 A | 4.2 ± 3.5 A |
| γ-Tocopherol (μg/g DW) | 21.2 ± 3.9 A | 25.4 ± 3.2 A |
| γ-Tocotrienol (μg/g DW) | 4.4 ± 1.5 A | 72.5 ± 10.8 B |
| Total tocols (μg/g DW) | 28.1 | 120.0 |
| γ-Oryzanol (μg/g DW) | 26.2 ± 4.1 A | 655.0 ± 30.1 B |
| Coixenolide (mg/g DW) | 4.0 ± 0.2 A | 8.7 ± 0.8 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Qin, L.; Liu, X.; Miao, S. Increases of Lipophilic Antioxidants and Anticancer Activity of Coix Seed Fermented by Monascus purpureus. Foods 2021, 10, 566. https://doi.org/10.3390/foods10030566
Zeng H, Qin L, Liu X, Miao S. Increases of Lipophilic Antioxidants and Anticancer Activity of Coix Seed Fermented by Monascus purpureus. Foods. 2021; 10(3):566. https://doi.org/10.3390/foods10030566
Chicago/Turabian StyleZeng, Haiying, Likang Qin, Xiaoyan Liu, and Song Miao. 2021. "Increases of Lipophilic Antioxidants and Anticancer Activity of Coix Seed Fermented by Monascus purpureus" Foods 10, no. 3: 566. https://doi.org/10.3390/foods10030566
APA StyleZeng, H., Qin, L., Liu, X., & Miao, S. (2021). Increases of Lipophilic Antioxidants and Anticancer Activity of Coix Seed Fermented by Monascus purpureus. Foods, 10(3), 566. https://doi.org/10.3390/foods10030566