Arthrospira platensis Extract: A Non-Invasive Strategy to Obtain Adjunct Attenuated Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Arthrospira Platensis Extraction Process
2.2. Bacterial Strains and Culture Conditions
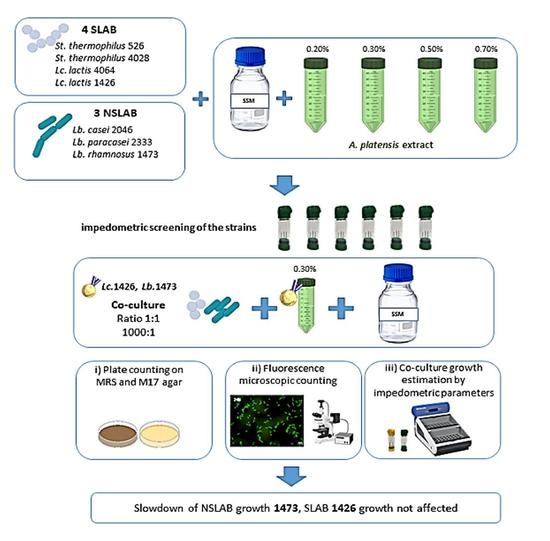
2.3. Experimental Design
2.4. Single Strain Growth Condition and Analysis
2.5. In Vitro Simulation of Selected SLAB and NSLAB
2.6. Impedometric Measurement
2.7. pH Measurement
2.8. Culture-Dependent Viable Counts of the Co-Cultures
2.9. Culture-Independent Viable Counts of the Co-Cultures
2.10. Statistical Analysis
3. Results and Discussion
3.1. Single Strains Selection
3.2. In Vitro Simulation of SLAB L. lactis 1426 and NSLAB Lb. rhamnosus 1473 Co-Culturing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; O’Toole, W.; Pot, B.; Vandamme, P.; Walter, J.; Watanabe, K.; Wuyts, S.; Felis, G.E.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Crow, V.; Curry, B.; Hayes, M. The ecology of non-starter lactic acid bacteria (NSLAB) and their use as adjuncts in New Zealand Cheddar. Int. Dairy J. 2001, 11, 275–283. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Pasquale, I.; De Angelis, M.; Buchin, S.; Calasso, M.; Fox, P.F.; Gobbetti, M. Manufacture of Italian Caciotta-type cheeses with adjuncts and attenuated adjuncts of selected non-starter lactobacilli. Int. Dairy J. 2011, 21, 254–260. [Google Scholar] [CrossRef]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus paracasei 4341 as adjunct culture to enhance flavor in short ripened Caciotta-type cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef]
- Upadhyay, V.K.; Huppertz, T.; Kelly, A.L.; McSweeney, P.L.H. Use of high pressure treatment to attenuate starter bacteria for use as adjuncts for Cheddar cheese manufacture. Innov. Food Sci. Emerg. Technol. 2007, 8, 485–492. [Google Scholar] [CrossRef]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Pasquale, I.; De Angelis, M.; Gobbetti, M. Accelerated ripening of Caciocavallo Pugliese cheese with attenuated adjuncts of selected nonstarter lactobacilli. J. Dairy Sci. 2012, 95, 4784–4795. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Levante, A.; Bancalari, E.; Sforza, S.; Bottesini, C.; Prandi, B.; De Filippis, F.; Ercolini, D.; Nocetti, M.; Gatti, M. The Interrelationship Between Microbiota and Peptides During Ripening as a Driver for Parmigiano Reggiano Cheese Quality. Front. Microbiol. 2020, 11, 14. [Google Scholar] [CrossRef]
- Peralta, G.H.; Bergamini, C.V.; Audero, G.; Páez, R.; Wolf, I.V.; Perotti, M.C.; Hynes, E.R. Spray-dried adjunct cultures of autochthonous non-starter lactic acid bacteria. Int. J. Food Microbiol. 2017, 255, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible Seaweeds and Spirulina Extracts for Food Application: In Vitro and In Situ Evaluation of Antimicrobial Activity towards Foodborne Pathogenic Bacteria. Foods 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Martelli, F.; Bernini, V.; Neviani, E.; Gatti, M. Bacteriostatic or bactericidal? Impedometric measurements to test the antimicrobial activity of Arthrospira platensis extract. Food Control 2020, 118, 107380. [Google Scholar] [CrossRef]
- Martelli, F.; Favari, C.; Mena, P.; Guazzetti, S.; Ricci, A.; Rio, D.D.; Lazzi, C.; Neviani, E.; Bernini, V. Antimicrobial and Fermentation Potential of Himanthalia elongata in Food Applications. Microorganisms 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancalari, E.; Bernini, V.; Bottari, B.; Neviani, E.; Gatti, M. Application of Impedance Microbiology for Evaluating Potential Acidifying Performances of Starter Lactic Acid Bacteria to Employ in Milk Transformation. Front. Microbiol. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levante, A.; Bancalari, E.; Tambassi, M.; Lazzi, C.; Neviani, E.; Gatti, M. Phenotypic Diversity of Lactobacillus casei Group Isolates as a Selection Criterion for Use as Secondary Adjunct Starters. Microorganisms 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancalari, E.; D’Incecco, P.; Savo Sardaro, M.L.; Neviani, E.; Pellegrino, L.; Gatti, M. Impedance microbiology to speed up the screening of lactic acid bacteria exopolysaccharide production. Int. J. Food Microbiol. 2019, 306, 10. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Santarelli, M.; Neviani, E.; Gatti, M. Natural whey starter for Parmigiano Reggiano: Culture-independent approach. J. Appl. Microbiol. 2010, 108, 1676–1684. [Google Scholar] [CrossRef]
- Gatti, M.; Bernini, V.; Lazzi, C.; Neviani, E. Fluorescence microscopy for studying the viability of micro-organisms in natural whey starters. Lett. Appl. Microbiol. 2006, 42, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Gatti, M. Survey on the community and dynamics of lactic acid bacteria in Grana Padano cheese. Syst. Appl. Microbiol. 2013, 36, 593–600. [Google Scholar] [CrossRef]
- Blaya, J.; Barzideh, Z.; LaPointe, G. Symposium review: Interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. J. Dairy Sci. 2018, 101, 3611–3629. [Google Scholar] [CrossRef]
- Guarrasi, V.; Sannino, C.; Moschetti, M.; Bonanno, A.; Di Grigoli, A.; Settanni, L. The individual contribution of starter and non-starter lactic acid bacteria to the volatile organic compound composition of Caciocavallo Palermitano cheese. Int. J. Food Microbiol. 2017, 259, 35–42. [Google Scholar] [CrossRef]
- Sarada, D.V.L.; Sreenath Kumar, C.; Rengasamy, R. Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: A novel and potent agent against drug resistant bacteria. World J. Microbiol. Biotechnol. 2011, 27, 779–783. [Google Scholar] [CrossRef]
- Bottari, B.; Levante, A.; Neviani, E.; Gatti, M. How the Fewest Become the Greatest. L. casei’s Impact on Long Ripened Cheeses. Front. Microbiol. 2018, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Mucchetti, G. Invited review: Microbial evolution in raw-milk, long-ripened cheeses produced using undefined natural whey starters. J. Dairy Sci. 2014, 97, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carafa, I.; Stocco, G.; Franceschi, P.; Summer, A.; Tuohy, K.M.; Bittante, G.; Franciosi, E. Evaluation of autochthonous lactic acid bacteria as starter and non-starter cultures for the production of Traditional Mountain cheese. Food Res. Int. 2019, 115, 209–218. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatnagar, A.K.; Srivastava, J.N. Antibacterial activity of crude extracts of Spirulina platensis and its structural elucidation of bioactive compound. J. Med. Plants Res. 2011, 5, 7043–7048. [Google Scholar] [CrossRef] [Green Version]
- Golmakani, M.-T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant Activities of Phycocyanin: A Bioactive Compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]




| Species | Strain | Origin | Growth Conditions | Reference Paper |
|---|---|---|---|---|
| Streptococcus thermophilus | 526 | Milk | M17, 42 °C anaerobiosis | [15] |
| Streptococcus thermophilus | 4028 | Curd | M17, 42 °C anaerobiosis | [15] |
| Lactococcus lactis | 4064 | Cheese | M17, 30 °C anaerobiosis | [15] |
| Lactococcus lactis | 1426 | Milk | M17, 30 °C anaerobiosis | [15] |
| Lacticaseibacillus casei | 2046 | Cheese | MRS, 37 °C anaerobiosis | [16] |
| Lacticaseibacillus paracasei | 2333 | Cheese | MRS, 37 °C anaerobiosis | [16] |
| Lacticaseibacillus rhamnosus | 1473 | Cheese | MRS, 37 °C anaerobiosis | [16] |
| Strains | A. platensis Extract Concentration | |||||
|---|---|---|---|---|---|---|
| 0% | 0.20% | 0.30% | 0.50% | 0.70% | ||
| S. thermophilus 526 | Lag | 1.03 b ± 0.01 | 1.39 b ± 0.08 | 1.91 a ± 0.21 | nd c ± 0.11 | nd c ± 0.18 |
| Rate | 5.40 a ± 0.15 | 5.30 a ± 0.28 | 3.77 b ± 0.01 | nd c ± 0.01 | nd c ± 0.01 | |
| yEnd | 29.35 a ± 0.18 | 23.29 b ± 0.08 | 20.59 c ± 0.20 | nd d ± 0.16 | nd d ± 0.01 | |
| S. thermophilus 4028 | Lag | <1 c ± 0.09 | 1.41 c ± 0.14 | 2.37 b ± 0.19 | 10.84 a ± 0.01 | nd d ± 0.01 |
| Rate | 4.06 a ± 0.28 | 3.98 a ± 0.18 | 2.2 b ± 0.01 | 0.29 c ± 0.15 | nd c ± 0.01 | |
| yEnd | 29.27 a ± 0.11 | 21.20 b ± 0.01 | 13.79 c ± 0.08 | 4.97 d ± 0.07 | nd e ± 0.01 | |
| L. lactis 1426 | Lag | 4.95 b ± 0.01 | 3.42 c ± 0.22 | 3.01 c ± 0.28 | 6.24a ± 0.12 | 32.93 d ± 0.08 |
| Rate | 5.79 a ± 0.08 | 2.97 b ± 0.01 | 1.95 c ± 0.01 | 0.64 d ± 0.01 | 0.09 e ± 0.01 | |
| yEnd | 27.39 a ± 0.21 | 19.90 b ± 0.12 | 18.18 c ± 0.15 | 10.23 d ± 0.01 | 3.06 e ± 0.28 | |
| L. lactis 4064 | Lag | 3.95 b ± 0.01 | 2.74 c ± 0.08 | 3.87 b ± 0.08 | 6.07 a ± 0.01 | nd d ± 0.01 |
| Rate | 6.11 a ± 0.28 | 2.15 b ± 0.01 | 1.41 c ± 0.01 | 0.39 d ± 0.25 | nd e ± 0.08 | |
| yEnd | 27.05 a ± 0.15 | 17.17 b ± 0.02 | 13.80 c ± 0.28 | 8.6 d ± 0.21 | nd e ± 0.01 | |
| Lb. casei 2046 | Lag | 1.28 b ± 0.26 | 1.41 b ± 0.01 | 2.37 c ± 0.06 | 10.84 a ± 0.01 | nd d ± 0.08 |
| Rate | 1.04 c ± 0.01 | 3.98 a ± 0.08 | 2.21 b ± 0.01 | 0.29 d ± 0.15 | nd e ± 0.01 | |
| yEnd | 29.52 a ± 0.13 | 21.20 b ± 0.11 | 13.79 c ± 0.07 | 4.97 d ± 0.08 | nd e ± 0.01 | |
| Lb. rhamnosus 1473 | Lag | 8.43 e ± 0.15 | 12.72 d ± 0.01 | 13.55 c ± 0.06 | 16.94 b ± 0.01 | 28.25 a ± 0.01 |
| Rate | 1.19 a ± 0.21 | 1.40 a ± 0.1 | 0.96 b ± 0.11 | 0.70 c ± 0.15 | 0.22 d ± 0.12 | |
| yEnd | 22.01 a ± 0.08 | 19.81 b ± 0.01 | 14.96 c ± 0.14 | 9.49 d ± 0.01 | 3.02 e ± 0.01 | |
| Lb. paracasei 2333 | Lag | 10.32 e ± 0.08 | 11.71 d ± 0.02 | 14.73 c ± 0.01 | 18.74 b ± 0.13 | 31.67 a ± 0.01 |
| Rate | 0.69 b ± 0.01 | 0.84 a ± 0.01 | 0.71 b ± 0.15 | 0.47 c ± 0.12 | 0.15 d ± 0.28 | |
| yEnd | 19.69 a ± 0.21 | 16.89 b ± 0.11 | 15.24 c ± 0.03 | 10.69 d ± 0.01 | 3.60 e ± 0.01 | |
| Co-Culture1_C | Co-Culture1_A | Co-Culture2_C | Co-Culture2_A | |
|---|---|---|---|---|
| Lag | 6.29 ± 0.02 a | 5.79 ± 0.08 b | 6.60 ± 0.06 a | 5.85 ± 0.03 b |
| Rate | 4.94 ± 0.01 a | 1.53 ± 0.02 b | 5.00 ± 0.05 a | 1.16 ± 0.04 b |
| yEnd | 26.71 ± 0.06 a | 15.44 ± 0.05 b | 25.95 ± 0.08 a | 13.78 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bancalari, E.; Martelli, F.; Bottari, B.; Neviani, E.; Gatti, M. Arthrospira platensis Extract: A Non-Invasive Strategy to Obtain Adjunct Attenuated Cultures. Foods 2021, 10, 588. https://doi.org/10.3390/foods10030588
Bancalari E, Martelli F, Bottari B, Neviani E, Gatti M. Arthrospira platensis Extract: A Non-Invasive Strategy to Obtain Adjunct Attenuated Cultures. Foods. 2021; 10(3):588. https://doi.org/10.3390/foods10030588
Chicago/Turabian StyleBancalari, Elena, Francesco Martelli, Benedetta Bottari, Erasmo Neviani, and Monica Gatti. 2021. "Arthrospira platensis Extract: A Non-Invasive Strategy to Obtain Adjunct Attenuated Cultures" Foods 10, no. 3: 588. https://doi.org/10.3390/foods10030588
APA StyleBancalari, E., Martelli, F., Bottari, B., Neviani, E., & Gatti, M. (2021). Arthrospira platensis Extract: A Non-Invasive Strategy to Obtain Adjunct Attenuated Cultures. Foods, 10(3), 588. https://doi.org/10.3390/foods10030588