Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives
Abstract
:1. Introduction
2. Materials and Methods
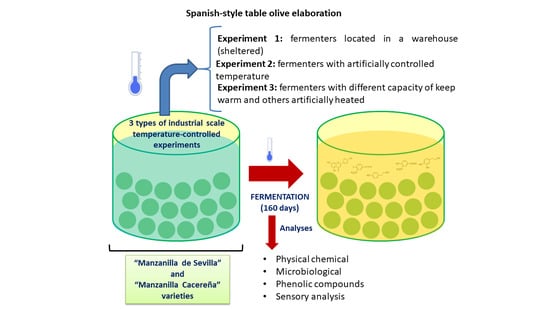
2.1. Experimental Design
2.2. Physical–Chemical Parameters
2.3. Phenolic Extraction and HPLC Analysis of the Phenolic Profile of Table Olives
2.4. Microbiological Analysis
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Thermal Treatments on Temperature within the Fermenter
3.2. Effects of Different Thermal Treatments on Microbiological Properties
3.3. Effects of Different Thermal Treatments on Physical–Chemical Properties
3.4. Effect of Thermal Treatments on the Phenolic Profile
3.5. Effect of Different Thermal Treatments on Sensory Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Compliance with Ethics Requirements
References
- IOC. World Table Olive and Olive Oil Production. 2020. Available online: http://www.internationaloliveoil.org (accessed on 20 December 2020).
- Martin-Vertedor, D.; Rodrigues, N.; Marx, I.M.G.; Veloso, A.C.A.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Fuentes de Mendoza, M.; De Miguel Gordillo, C.; Marín Expósito, J.; Sánchez Casas, J.; Martínez Cano, M.; Martín Vertedor, D.; Franco Baltasar, M.N. Chemical composition of virgin olive oils according to the ripening in olives. Food Chem. 2013, 141, 2575–2581. [Google Scholar] [CrossRef]
- Fuentes, M.; De Miguel, C.; Ranalli, A.; Franco, M.N.; Martínez, M.; Martín-Vertedor, D. Chemical composition and sensory evaluation of virgin olive oils from “Morisca” and ‘Manzanilla de Sevilla’ olive varieties. Grasas Aceites 2015, 66, 1–16. [Google Scholar]
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and innovative approaches to obtain virgin olive oils with a higher level of bioactive constituents. In Olive and Olive Oil Bioactive Constituents; AOCS Press (Elsevier Inc.): Urbana, IL, USA, 2015; pp. 179–215. [Google Scholar]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Morozzi, G.; Fabiani, R. Oleuropein inhibits tumour growth and metastases dissemination in ovariectomised nude mice with MCF-7 human breast tumour xenografts. J. Funct. Foods 2014, 8, 269–273. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; López, O.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Bautista-Gallego, J.; Arroyo-López, F.N.; Romero-Gil, V.; Jiménez-Díaz, R.; Garrido-Fernández, A.; García-García, P. Table olive fermentation with multifunctional Lactobacillus pentosus strains. Food Control 2013, 34, 96–105. [Google Scholar] [CrossRef]
- Benincasa, C.; Muccilli, S.; Amenta, M.; Perri, E.; Romeo, F.V. Phenolic trend and hygienic quality of green table olives fermented with Lactobacillus plantarum starter culture. Food Chem. 2015, 186, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Martín, A.; Aranda, E.; Pérez-Nevado, F.; Córdoba, M.G. Identification and characterization of yeast isolated from the elaboration of seasoned green table olives. Food Microbiol. 2007, 24, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- Ruíz-Moyano, S.; Martín, A.; Hernández, A.; Casquete-Palencia, R. Nuevas Tecnologías en la elaboración de la aceituna de mesa. In La Agricultura y la Ganadería Extremeñas, Informe 2009; Caja de Ahorros de Badajoz: Badajoz, Spain, 2010; pp. 105–119. ISBN 978-84-889-5699-1. [Google Scholar]
- Quintana, M.D.; García, P.G.; Fernández, A.G. Establishment of conditions for green table olive fermentation at low temperature. Int. J. Food Microbiol. 1999, 51, 133–143. [Google Scholar] [CrossRef]
- López, F.N.A.; Quintana, M.D.; Fernández, A.G. Use of the generalized z-value concept to study the effects of temperature, NaCl concentration and pH on Pichia anomala, a yeast related to table olive fermentation. Int. J. Food Microbiol. 2006, 106, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bañegil, M.; Pérez-Nevado, F.; Montaño, A.; Pleite, R.; Martín-Vertedor, D. The effect of olive fruit maturation in Spanish style fermentation with a controlled temperature. LWT Food Sci. Technol. 2018, 91, 40–47. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Reguart, C.; Bordons, A.; Rozès, N. Influence of fruit ripeness and salt concentration on the microbial processing of Arbequina table olives. Food Microbiol. 2009, 26, 827–833. [Google Scholar] [CrossRef]
- Lucena-Padrós, H.; Ruiz-Barba, J.L. Microbial biogeography of Spanish-style green olive fermentations in the province of Seville, Spain. Food Microbiol. 2019, 82, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; García-García, P.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Arroyo-López, F.N. Evaluating the individual effects of temperature and salt on table olive related microorganisms. Food Microbiol. 2013, 33, 178–184. [Google Scholar] [CrossRef] [PubMed]
- de Florio Ramírez, E.; Bellido-Valencia, O.; Medina-Marroquín, L.A.; Vásquez-Chicata, A. Effect of sodium hydroxide concentration, temperature and fruit size on the processing time of sevillian-style green table olives (Olea Europaea L.). Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology. Food Ind. 2019, 20, 21–28. [Google Scholar]
- Kiai, H.; Hafidi, A. Chemical composition changes in four green olive cultivars during spontaneous fermentation. Lwt Food Sci. Technol. 2014, 57, 663–670. [Google Scholar] [CrossRef]
- Pereira, L.E.; Ramalhosa, E.; Borges, A.; Pereira, J.A.; Baptista, P. Yeast dynamics during the natural fermentation process of table olives (Negrinha de Freixo cv.). Food Microbiol. 2015, 46, 582–586. [Google Scholar] [CrossRef] [Green Version]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Pistarino, E.; Aliakbarian, B.; Casazza, A.A.; Paini, M.; Cosulich, M.E.; Perego, P. Combined effect of starter culture and temperature on phenolic compounds during fermentation of Taggiasca black olives. Food Chem. 2013, 138, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Asociación para la Promoción de las Aceitunas Sevillanas de las Variedades Manzanilla y Gordal (APAS). 2015. Available online: https://serraniasuroeste.org/documentos/Caracterizacion_organoleptica.pdf (accessed on 11 November 2020).





| (A) Experiment 1 | ||||
| Fermentation Day | Heat Treatment | LAB | Yeasts and Mold | Mesophile |
| 5 | Control | 1.24 ± 0.21 n.s. | 1.20 ± 0.25 n.s. | 1.42 ± 0.33 n.s. |
| Sheltered | 1.29 ± 0.86 | 1.53 ± 0.32 | 1.22 ± 0.23 | |
| 20 | Control | 2.25 ± 0.77 a | 3.45 ± 0.24 n.s. | 2.52 ± 0.28 n.s. |
| Sheltered | 3.37 ± 0.05 b | 3.38 ± 0.49 | 3.03 ± 0.11 | |
| 60 | Control | 3.99 ± 0.14 a | 3.54 ± 0.03 n.s. | 3.04 ± 0.19 a |
| Sheltered | 5.33 ± 0.14 b | 3.11 ± 0.12 | 3.79 ± 0.13 b | |
| 100 | Control | 3.85 ± 0.24 a | 4.07 ± 0.54 b | 3.62 ± 0.11 a |
| Sheltered | 5.28 ± 0.12 b | 3.07 ± 0.54 a | 4.29 ± 0.14 b | |
| 140 | Control | 3.12 ± 0.12 a | 4.79 ± 0.13 b | 4.55 ± 0.12 a |
| Sheltered | 5.01 ± 0.22 b | 4.07 ± 0.21 a | 5.42 ± 0.22 b | |
| 160 | Control | 2.80 ± 0.11 a | 5.01 ± 0.11 b | 5.05 ± 0.11 a |
| Sheltered | 4.51 ± 0.11 b | 4.02 ± 0.12 a | 6.65 ± 0.15 b | |
| (B) Experiment 2 | ||||
| Fermentation Day | Heat Treatment | LAB | Yeasts and Mold | Mesophile |
| 5 | Control | 1.11 ± 0.11 a | 1.23 ± 0.13 a | 1.31 ± 0.23 n.s. |
| 20 | 2.12 ± 0.13 b | 1.61 ± 0.11 c | 1.21 ± 0.10 | |
| 22 | 2.11 ± 0.12 b | 1.32 ± 0.14 b | 1.22 ± 0.14 | |
| 24 | 2.23 ± 0.13 b | 1.50 ± 0.14 b | 1.32 ± 0.13 b | |
| 20 | Control | 3.15 ± 0.24 a | 4.22 ± 0.22 a | 3.22 ± 0.23 a |
| 20 | 5.82 ± 0.12 b | 4.01 ± 0.24 a | 3.76 ± 0.11 b | |
| 22 | 6.05 ± 0.22 c | 4.42 ± 0.15 b | 3.83 ± 0.12 b | |
| 24 | 6.33 ± 0.13 c | 4.53 ± 0.15 b | 3.81 ± 0.22 b | |
| 60 | Control | 3.09 ± 0.11 a | 3.10 ± 0.10 a | 3.21 ± 0.11 a |
| 20 | 5.31 ± 0.13 b | 4.11 ± 0.11 b | 4.02 ± 0.14 b | |
| 22 | 6.15 ± 0.21 c | 4.31 ± 0.14 c | 4.23 ± 0.14 b | |
| 24 | 6.54 ± 0.22 d | 4.42 ± 0.14 c | 4.41 ± 0.21 c | |
| 100 | Control | 3.42 ± 0.21 a | 4.12 ± 0.23 ns | 3.42 ± 0.13 a |
| 20 | 5.51 ± 0.22 b | 4.22 ± 0.21 | 4.32 ± 0.13 b | |
| 22 | 6.15 ± 0.31 c | 4.42 ± 0.13 | 4.11 ± 0.11 b | |
| 24 | 6.51 ± 0.32 d | 4.53 ± 0.32 | 4.32 ± 0.23 b | |
| 140 | Control | 3.02 ± 0.22 a | 4.12 ± 0.12 a | 4.35 ± 0.12 a |
| 20 | 6.4 ± 0.21 c | 4.5 ± 0.11 b | 5.21 ± 0.21 b | |
| 22 | 6.23 ± 0.11 b | 4.64 ± 0.12 c | 5.12 ± 0.21 b | |
| 24 | 6.44 ± 0.21 c | 4.72 ± 0.21 c | 5.71 ± 0.22 c | |
| 160 | Control | 2.2 ± 0.1 a | 3.4 ± 0.1 a | 5.3 ± 0.1 a |
| 20 | 4.3 ± 0.1 b | 4.2 ± 0.1 b | 6.3 ± 0.2 b | |
| 22 | 4.3 ± 0.1 b | 4.7 ± 0.1 c | 6.6 ± 0.2 b | |
| 24 | 4.4 ± 0.1 b | 4.2 ± 0.1 b | 6.7 ± 0.2 b | |
| (C) Experiment 3 | ||||
| Fermentation Day | Heat Treatment | LAB | Yeasts and Mold | Mesophile |
| 5 | Control | 1.52 ± 0.21 n.s. | 1.35 ± 0.23 n.s. | 1.45 ± 0.14 n.s. |
| Buried | 2.02 ± 0.23 | 1.07 ± 0.24 | 1.54 ± 0.53 | |
| Isolated | 1.63 ± 0.22 | 1.33 ± 0.41 | 1.73 ± 0.42 | |
| 20 | Control | 3.32 ± 0.12 n.s. | 4.11 ± 0.31 n.s. | 3.23 ± 0.22 n.s. |
| Buried | 3.22 ± 0.24 | 3.01 ± 0.42 | 3.01 ± 0.58 | |
| Isolated | 3.31 ± 0.32 | 3.06 ± 0.32 | 4.02 ± 0.31 | |
| 60 | Control | 3.73 ± 0.10 a | 3.11 ± 0.23 a | 3.33 ± 0.23 a |
| Buried | 3.84 ± 0.22 b | 3.91 ± 0.22 b | 4.41 ± 0.11 b | |
| Isolated | 4.92 ± 0.22 c | 4.01 ± 0.22 c | 4.23 ± 0.24 b | |
| 100 | Control | 4.23 ± 0.22 a | 4.53 ± 0.12 n.s. | 4.31 ± 0.12 n.s. |
| Buried | 5.02 ± 0.12 b | 4.21 ± 0.11 | 4.22 ± 0.21 | |
| Isolated | 6.01 ± 0.12 c | 4.06 ± 0.31 | 4.11 ± 0.12 | |
| 140 | Control | 3.56 ± 0.21 a | 4.02 ± 0.11 a | 4.15 ± 0.13 n.s. |
| Buried | 5.02 ± 0.11 b | 4.51 ± 0.21 c | 4.03 ± 0.11 | |
| Isolated | 5.93 ± 0.24 c | 4.32 ± 0.21 b | 4.13 ± 0.44 | |
| 160 | Control | 2.31 ± 0.11 a | 3.01 ± 0.11 a | 5.01 ± 0.23 a |
| Buried | 3.82 ± 0.12 c | 3.72 ± 0.12 b | 6.02 ± 0.22 b | |
| Isolated | 3.53 ± 0.13 b | 3.61 ± 0.12 b | 6.12 ± 0.11 b | |
| (A) Experiment 1 | ||||||
| Heat Treatment | Hydroxytyrosol | Tyrosol | Oleuropein | Apigenin | Luteolin | Luteolin-7-O-Glucoside |
| Control | 551 ± 55 b | 126 ± 16 b | 108 ± 17 n.s. | 1.3 ± 0.4 n.s. | 8.4 ± 3.1 a | 2.6 ± 0.6 n.s. |
| Sheltered | 484 ± 84 a | 104 ± 14 a | 99 ± 19 | 1.3 ± 1.1 | 13.9 ± 3.8 b | 2.3 ± 0.7 |
| Vanillin | Vanillic Acid | PB1 | PB2 | Epicatechin | Catechin | |
| Control | 2.8 ± 0.8 n.s. | 4.1 ± 0.6 n.s. | 13.1 ± 3.7 n.s. | 2.4 ± 0.4 n.s. | 6.7 ± 0.7 b | 6.6 ± 1.5 n.s. |
| Sheltered | 2.4 ± 0.7 | 3.4 ± 2.5 | 14.8 ± 6.3 | 2.1 ± 0.6 | 4.6 ± 1.9 a | 7.7 ± 2.7 |
| (B) Experiment 2 | ||||||
| Heat treatment | Hydroxytyrosol | Tyrosol | Oleuropein | Apigenin | Luteolin | Verbascoside |
| Control | 1026 ± 50 n.s. | 198 ± 26 c | 124 ± 8 c | 0.6 ± 0.1 b | 4.0 ± 0.2 a | 1.7 ± 0.8 b |
| 20 | 963 ± 100 | 162 ± 22 b | 100 ± 6 a,b | 0.4 ± 0.1 a | 4.2 ± 0.2 b | 0.8 ± 0.4 a |
| 22 | 963 ± 82 | 144 ± 16 a | 109 ± 7 b | 0.8 ± 0.1 c | 5.7 ± 0.2 c | 0.7 ± 0.3 a |
| 24 | 929 ± 82 | 148 ± 32 a | 88 ± 8 a | 0.4 ± 0.1 a | 4.4 ± 0.2 b | 1.0 ± 0.1 a |
| Vanillin | Vanillic Acid | PB1 | PB2 | Catechin | Epicatechin | |
| Control | 8.2 ± 1.1 n.s. | 3.3 ± 0.2 n.s. | 15 ± 2 b | 2.8 ± 0.4 b | 7.3 ± 0.5 n.s. | 6.0 ± 0.8 n.s. |
| 20 | 8.3 ± 0.1 | 3.5 ± 0.4 | 15 ± 1 b | 2.2 ± 0.3 a | 8.0 ± 0.3 | 5.9 ± 0.5 |
| 22 | 10.9 ± 0.2 | 4.3 ± 0.3 | 15 ± 1 b | 2.7 ± 0.2 a,b | 7.9 ± 0.4 | 6.6 ± 0.7 |
| 24 | 11.6 ± 0.5 | 4.0 ± 0.3 | 10 ± 1 a | 2.6 ± 0.2 a,b | 7.9 ± 0.5 | 6.1 ± 0.3 |
| (C) Experiment 3 | ||||||
| Heat Treatment | Hydroxytyrosol | Tyrosol | Oleuropein | Apigenin | Luteolin | o-Vanillin |
| Buried | 915 ± 245 n.s. | 153 ± 39 n.s. | 142 ± 39 a,b | n.q. | 4.7 ± 1.9 n.s. | 14.1 ± 3.6 b |
| Older | 859 ± 175 | 141 ± 28 | 115 ± 33 a | n.q. | 3.7 ± 0.7 | 9.3 ± 6.6 a |
| Isolated | 934 ± 175 | 151 ± 32 | 159 ± 32 b | 0.60 ± 0.27 | 5.1 ± 1.0 | 16.0 ± 5.3 b |
| Vanillin | Vanillic Acid | PB1 | PB2 | Epicatechin | Catechin | |
| Buried | 5.0 ± 1.5 a | 4.2 ± 1.5 n.s. | 17.1 ± 5.3 n.s. | 2.09 ± 0.27 b | 4.8 ± 1.4 n.s. | 7.9 ± 2.1 n.s. |
| Older | 4.7 ± 1.3 a | 3.5 ± 1.4 | 14.4 ± 5.2 | 1.75 ± 0.13 a | 5.57 ± 0.93 | 6.6 ± 2.0 |
| Isolated | 6.8 ± 1.6 b | 4.3 ± 1.2 | 17.8 ± 5.6 | 2.22 ± 0.44 b | 5.01 ± 0.98 | 8.5 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; Arias-Calderón, R.; Pérez-Nevado, F. Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives. Foods 2021, 10, 666. https://doi.org/10.3390/foods10030666
Martín-Vertedor D, Schaide T, Boselli E, Martínez M, Arias-Calderón R, Pérez-Nevado F. Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives. Foods. 2021; 10(3):666. https://doi.org/10.3390/foods10030666
Chicago/Turabian StyleMartín-Vertedor, Daniel, Thaís Schaide, Emanuele Boselli, Manuel Martínez, Rocío Arias-Calderón, and Francisco Pérez-Nevado. 2021. "Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives" Foods 10, no. 3: 666. https://doi.org/10.3390/foods10030666
APA StyleMartín-Vertedor, D., Schaide, T., Boselli, E., Martínez, M., Arias-Calderón, R., & Pérez-Nevado, F. (2021). Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives. Foods, 10(3), 666. https://doi.org/10.3390/foods10030666