Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements
Abstract
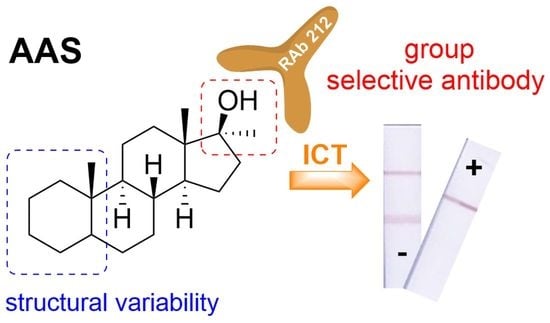
:1. Introduction
2. Materials and Apparatus
2.1. Immunoreagencies
2.2. Chemicals
2.3. Material for ICT
2.4. Buffers
2.5. Apparatus and Software
3. Experimental Section
3.1. Preparation of Conjugates of GAR-AuNPs and RAb-AuNPs
3.2. Preparation of Samples and Matrices
3.3. Preparation of ICT Strips
3.4. Open Format
3.5. Closed Format
3.6. Test Evaluation
3.7. Cross Interactions
4. Results and Discussion
4.1. Immunochromatographic Test
4.2. Indirect Format
4.3. Direct Format
4.4. Spiked Food Supplements Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geyer, H.; Parr, M.K.; Mareck, U.; Reinhart, U.; Schrader, Y.; Schanzer, W. Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids-results of an international study. Int. J. Sports Med. 2004, 25, 124–129. [Google Scholar] [CrossRef]
- De Cock, K.J.; Delbeke, F.T.; Van Eenoo, P.; Desmet, N.; Roels, K.; De Backer, P. Detection and determination of anabolic steroids in nutritional supplements. J. Pharm. Biomed. Anal. 2001, 25, 843–852. [Google Scholar] [CrossRef]
- Walpurgis, K.; Thomas, A.; Geyer, H.; Mareck, U.; Thevis, M. Dietary supplement and food tontaminations and their implications for doping controls. Foods 2020, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanz, J.M.; Sospedra, I.; Ortiz, C.M.; Baladia, E.; Gil-Izquierdo, A.; Ortiz-Moncada, R. Intended or unintended doping? A review of the presence of doping substances in dietary supplements used in sports. Nutrients 2017, 9, 1093. [Google Scholar] [CrossRef] [Green Version]
- Baume, N.; Mahler, N.; Kamber, M.; Mangin, P.; Saugy, M. Research of stimulants and anabolic steroids in dietary supplements. Scand. J. Med. Sci. Sports 2006, 16, 41–48. [Google Scholar] [CrossRef]
- Jurášek, M.; Göselová, S.; Mikšátková, P.; Holubová, B.; Vyšatová, E.; Kuchař, M.; Fukal, L.; Lapčík, O.; Drašar, P. Highly sensitive avidin-biotin ELISA for detection of nandrolone and testosterone in dietary supplements. Drug Test. Anal. 2017, 9, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Huml, L.; Havlová, D.; Longin, O.; Staňková, E.; Holubová, B.; Kuchař, M.; Prokudina, E.; Rottnerová, Z.; Zimmermann, T.; Drašar, P.; et al. Stanazolol derived ELISA as a sensitive forensic tool for the detection of multiple 17α-methylated anabolics. Steroids 2020, 155, 108550. [Google Scholar] [CrossRef]
- Fojtíková, L.; Fukal, L.; Blažková, M.; Sýkorová, S.; Kuchař, M.; Mikšátková, P.; Lapčík, O.; Holubová, B. Development of enzyme-linked immunosorbent assay for determination of boldenone in dietary supplements. Food Anal. Methods 2016, 9, 3179–3186. [Google Scholar] [CrossRef]
- Sýkorová, S.; Fojtíková, L.; Kuchař, M.; Mikšátková, P.; Karamonová, L.; Fukal, L.; Lapčík, O.; Holubová, B. Sensitive enzyme immunoassay for screening methandienone in dietary supplements. Food Addit. Contam. Part. A 2018, 35, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Petrakova, A.V.; Zherdev, A.V.; Dzantiev, B.B. “Multistage in one touch” design with a universal labelling conjugate for high-sensitive lateral flow immunoassays. Biosens. Bioelectron. 2016, 86, 575–579. [Google Scholar] [CrossRef]
- Agnamey, P.; Sarfati, C.; Pinel, C.; Rabodoniriina, M.; Kapel, N.; Dutoit, E.; Garnaud, C.; Diouf, M.; Garin, J.F.; Totet, A.; et al. Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: A blind multicenter trial. J. Clin. Microbiol. 2011, 49, 1605–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.-W.; Wang, Y.-B.; Fang, L.-L.; Wu, Y.; Yang, L.; Chen, J.-Y.; Ge, S.-X.; Zhang, J.; Xiong, Y.-Z.; Deng, X.-M.; et al. Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Anal. Chem. 2015, 87, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the numbers of gold nanoparticles in the test zone of lateral flow immunoassay strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Nicol, T.; Lefeuvre, C.; Serri, O.; Pivert, A.; Joubaud, F.; Dubée, V.; Kouatchet, A.; Ducancelle, A.; Lunel-Fabiani, F.; Le Guillou-Guillemette, H. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 2020, 129, 104511. [Google Scholar] [CrossRef] [PubMed]
- Karakus, C.; Salih, B.A. Comparison of the lateral flow immunoassays (LFIA) for the diagnosis of Helicobacter pylori infection. J. Immunol. Methods 2013, 396, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wiriyachaiporn, S.; Howarth, P.H.; Bruce, K.D.; Dailey, L.A. Evaluation of a rapid lateral flow immunoassay for Staphylococcus aureus detection in respiratory samples. Diagnostic Microbiology and Infectious Disease 2013, 75, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, B.; Fang, J.; Zhi, A.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.; Zhang, M. Recombinase polymerase amplification (RPA) combined with lateral flow immunoassay for rapid detection of Salmonella in food. Foods 2020, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, N.; Nara, S. Lateral flow assay for rapid detection of Staphylococcus aureus enterotoxin A in milk. Microchem. J. 2018, 137, 435–442. [Google Scholar] [CrossRef]
- Zvereva, E.A.; Hendrickson, O.D.; Zherdev, A.V.; Dzantiev, B.B. Immunochromatographic tests for the detection of microcystin-LR toxin in water and fish samples. Anal. Methods 2020, 12, 392–400. [Google Scholar] [CrossRef]
- Pan, M.; Ma, T.; Yang, J.; Li, S.; Liu, S.; Wang, S. Development of lateral flow immunochromatographic assays using colloidal Au sphere and nanorods as signal marker for the determination of zearalenone in cereals. Foods 2020, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Blažková, M.; Micková-Holubová, B.; Rauch, P.; Fukal, L. Immunochromatographic colloidal carbon-based assay for detection of methiocarb in surface water. Biosens. Bioelectron. 2009, 25, 753–758. [Google Scholar] [CrossRef]
- Bayoumy, S.; Hyytiä, H.; Leivo, J.; Talha, S.M.; Huhtinen, K.; Poutanen, M.; Hynninen, J.; Perheentupa, A.; Lamminmäki, U.; Gidwani, K.; et al. Glycovariant-based lateral flow immunoassay to detect ovarian cancer–associated serum CA125. Commun. Biol. 2020, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.C.; Chou, C.C.; Yang, Y.Q.; Wei-Kai, T.; Wang, Y.T.; Chan, Y.H. Multiplexed detection of tumor markers with multicolor polymer dot-based immunochromatography test strip. Anal. Chem. 2018, 90, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Berlina, A.N.; Bartosh, A.V.; Zherdev, A.V.; Xu, C.L.; Dzantiev, B.B. Development of immunochromatographic assay for determination of tetracycline in human serum. Antibiotics 2018, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickson, O.D.; Zvereva, E.A.; Shanin, I.A.; Zherdev, A.V.; Tarannum, N.; Dzantiev, B.B. Highly sensitive immunochromatographic detection of antibiotic ciprofloxacin in milk. Appl. Biochem. Microbiol. 2018, 54, 670–676. [Google Scholar] [CrossRef]
- Fojtíková, L.; Šuláková, A.; Blažková, M.; Holubová, B.; Kuchař, M.; Mikšátková, P.; Lapčík, O.; Fukal, L. Lateral flow immunoassay and enzyme linked immunosorbent assay as effective immunomethods for the detection of synthetic cannabinoid JWH-200 based on the newly synthesized hapten. Toxicol. Rep. 2018, 5, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wennig, R.; Moeller, M.R.; Haguenoer, J.M.; Marocchi, A.; Zoppi, F.; Smith, B.L.; de la Torre, R.; Carstensen, C.A.; Goerlach-Graw, A.; Schaeffler, J.; et al. Development and evaluation of immunochromatographic rapid tests for screening of cannabinoids, cocaine, and opiates in urine. J. Anal. Toxicol. 1998, 22, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiao, W.; Kong, H.; Cheng, J.J.; Yan, X.; Zhang, M.L.; Wang, Q.G.; Qu, H.H.; Zhao, Y.A. Highly sensitive immunochromatographic strip test for rapid and quantitative detection of Saikosaponind. Molecules 2018, 23, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Guo, L.; Yu, M.; Zhao, H. The application of a lateral flow immunographic assay to rapidly test for dexamethasone in commercial facial masks. Anal. Bioanal. Chem. 2019, 411, 5703–5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsonova, J.V.; Safronova, V.A.; Osipov, A.P. Pretreatment-free lateral flow enzyme immunoassay for progesterone detection in whole cows milk. Talanta 2015, 132, 685–689. [Google Scholar] [CrossRef]
- Oh, H.K.; Kim, J.W.; Kim, J.M.; Kim, M.G. High sensitive and broad-range detection of cortisol in human saliva using a trap lateral flow immunoassay (trapLFI) sensor. Analyst 2018, 143, 3883–3889. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Peng, C.F.; Jin, Z.Y.; Xu, C.L. Development and evaluation of a rapid lateral flow immunochromatographic strip assay for screening 19-nortestosterone. Biomed. Chromatogr. 2007, 21, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Wang, Z.H.; Dou, L.N.; Zhao, B.X.; He, Y.X.; Wang, J.L.; Sun, J.; Li, T.; Zhang, D.H. An innovative immunochromatography assay for highly sensitive detection of 17 beta-estradiol based on an indirect probe strategy. Sens. Actuators B Chem. 2019, 289, 48–55. [Google Scholar] [CrossRef]
- Lou, S.; Ye, J.Y.; Li, K.Q.; Wu, A.G. A gold nanoparticle-based immunochromatographic assay: The influence of nanoparticulate size. Analyst 2012, 137, 1174–1181. [Google Scholar] [CrossRef]
- Razo, S.C.; Panferova, N.A.; Panferov, V.G.; Safenkova, I.V.; Drenova, N.V.; Varitsev, Y.A.; Zherdev, A.V.; Pakina, E.N.; Dzantiev, B.B. Enlargement of gold nanoparticles for sensitive immunochromatographic diagnostics of potato Brown Rot. Sensors 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blažková, M.; Rauch, P.; Fukal, L. Strip-based immunoassay for rapid detection of thiabendazole. Biosens. Bioelectron. 2010, 25, 2122–2128. [Google Scholar] [CrossRef]
- Hua, X.D.; Yang, J.F.; Wang, L.M.; Fang, Q.K.; Zhang, G.P.; Liu, F.Q. Development of an enzyme linked immunosorbent assay and an immunochromatographic assay for detection of organophosphorus pesticides in different agricultural products. PLoS ONE 2012, 7, e53099. [Google Scholar] [CrossRef] [Green Version]
- Stepan, R.; Cuhra, P.; Barsova, S. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection for the determination of anabolic steroids and related compounds in nutritional supplements. Food Addit. Contam. Part. A 2008, 25, 557–565. [Google Scholar] [CrossRef]
- Huang, X.L.; Aguilar, Z.P.; Xu, H.Y.; Lai, W.H.; Xiong, Y.H. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.A.; Kim, M.Y.; Lee, Y.T.; Hammock, B.D.; Lee, H.S. Importance of membrane selection in the development of immunochromatographic assays for low-molecular weight compounds. Anal. Chim. Acta 2012, 757, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Jones, K. FUSION 5: A new platform for lateral flow immunoassay tests. Lateral Flow Immunoass. 2009, 115–129. [Google Scholar] [CrossRef]
- Girotti, S.; Eremin, S.; Montoya, A.; Moreno, M.J.; Caputo, P.; D’Elia, M.; Ripani, L.; Romolo, F.S.; Maiolini, E. Development of a chemiluminescent ELISA and a colloidal gold-based LFIA for TNT detection. Anal. Bioanal. Chem. 2010, 396, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Šuláková, A.; Fojtíková, L.; Holubová, B.; Bártová, K.; Lapčík, O.; Kuchař, M. Two immunoassays for the detection of 2C-B and related hallucinogenic phenethylamines. J. Pharmacol. Toxicol. Methods 2019, 95, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pantaleón, C.; Wichers, J.; Abad-Somovilla, A.; van Amerongen, A.; Abad-Fuentes, A. Development of an immunochromatographic assay based on carbon nanoparticles for the determination of the phytoregulator forchlorfenuron. Biosens. Bioelectron. 2013, 42, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Song, S.; Wu, X.; Liu, L.; Kuang, H.; Xiao, J.; Xu, C. Determination of robenidine in shrimp and chicken samples using the indirect competitive enzyme-linked immunosorbent assay and immunochromatographic strip assay. Analyst 2021, 146, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Z.; Guo, L.; Xu, X.; Wu, A.; Kuang, H.; Sun, L.; Song, S.; Xu, C. Sensitive lateral flow immunoassay for the residues of imidocarb in milk and beef samples. ACS Omega 2021, 6, 2559–2569. [Google Scholar] [CrossRef]
- Kong, N.; Song, S.; Peng, J.; Liu, L.; Kuang, H.; Xu, C. Sensitive, fast, and specific immunoassays for methyltestosterone detection. Sensors 2015, 15, 10059–10073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zou, S.; Xing, C.; Song, S.; Liu, L.; Xu, C. Preparation of a monoclonal antibody against testosterone and its use in development of an immunochromatographic assay. Food Agric. Immunol. 2016, 27, 547–558. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Song, S.; Feng, M.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Holubová, B.; Mikšátková, P.; Kuchař, M.; Karamonová, L.; Lapčík, O.; Fukal, L. Immunochemical techniques for anabolic androgenic steroid: Matrix effects study for food supplements. Eur. Food Res. Technol. 2019, 245, 1011–1019. [Google Scholar] [CrossRef]
- Hernandez-Guerra, A.I.; Tapia, J.; Menendez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic steroids abuse: Case report and literature review. Forensic. Sci. Res. 2019, 4, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gymporn.cz/sestavovani-kury (accessed on 17 December 2020).
- Steroidové Cykly. Available online: http://steroidy.szm.com/cykly.html (accessed on 17 December 2020).
- Potravinářská Inspekce Zakázala Doplněk Stravy z USA s Šesti Nepovolenými Látkami Včetně Anabolických Steroidů a Léčiv. Available online: https://www.szpi.gov.cz/clanek/potravinarska-inspekce-zakazala-doplnek-stravy-z-usa-s-sesti-nepovolenymi-latkami-vcetne-anabolickych-steroidu-a-leciv.aspx?q=JmNobnVtPTEmaGw9dGVzdG9zdGVyb24%3d (accessed on 17 December 2020).
- Potravinářská Inspekce Zakázala Potravinu s Anabolickým Steroidem. Available online: https://www.szpi.gov.cz/clanek/potravinarska-inspekce-zakazala-potravinu-s-anabolickym-steroidem.aspx (accessed on 17 December 2020).
- Průběžné Výsledky Kontroly Doplňků Pro Sportovce: Největší Problémy Jsou s Označováním, Anabolika Zatím Pouze ve Dvou Výrobcích. Available online: https://www.szpi.gov.cz/clanek/prubezne-vysledky-kontroly-doplnku-pro-sportovce-nejvetsi-problemy-jsou-s-oznacovanim-anabolika-zatim-pouze-ve-dvou-vyrobcich.aspx?q=JmhsPWFuYWJvbGlrYcKo (accessed on 17 December 2020).





| Parameter | Opened Format | Closed Format |
|---|---|---|
| Concentration of DAG in CL | 100 μg/mL | 100 μg/mL |
| Concentration of RSA/ST-3 in TL | 100 μg/mL | 300 μg/mL |
| Amount of GAR-AuNPs | 5 μL | 3 μL |
| Amount of RAb 212 | 2 μL | 2 μL |
| Membrane * | AE 98 | AE 98 |
| Membrane pad * | HF000MC100 | HF000MC100 |
| Sample pad * | GFCP 103000 | Grade 1281 |
| Conjugation pad * | NA a | Grade 6615 |
| Absorption pad * | CFSP 223000 | Grade 320 |
| Drying buffer | NA a | 0.2 mol/L borate buffer—0.1%, BSA—3%, trehalose—1% Tween 20 |
| Reaction buffer | 0.1 mol/L borate buffer—1%, BSA—1%, PEG—1% Tween 20 | 0.1 mol/L borate buffer—1%, BSA—1%, PEG—1% Tween 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubová, B.; Kubešová, P.; Huml, L.; Vlach, M.; Lapčík, O.; Jurášek, M.; Fukal, L. Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements. Foods 2021, 10, 741. https://doi.org/10.3390/foods10040741
Holubová B, Kubešová P, Huml L, Vlach M, Lapčík O, Jurášek M, Fukal L. Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements. Foods. 2021; 10(4):741. https://doi.org/10.3390/foods10040741
Chicago/Turabian StyleHolubová, Barbora, Pavla Kubešová, Lukáš Huml, Miroslav Vlach, Oldřich Lapčík, Michal Jurášek, and Ladislav Fukal. 2021. "Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements" Foods 10, no. 4: 741. https://doi.org/10.3390/foods10040741
APA StyleHolubová, B., Kubešová, P., Huml, L., Vlach, M., Lapčík, O., Jurášek, M., & Fukal, L. (2021). Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements. Foods, 10(4), 741. https://doi.org/10.3390/foods10040741