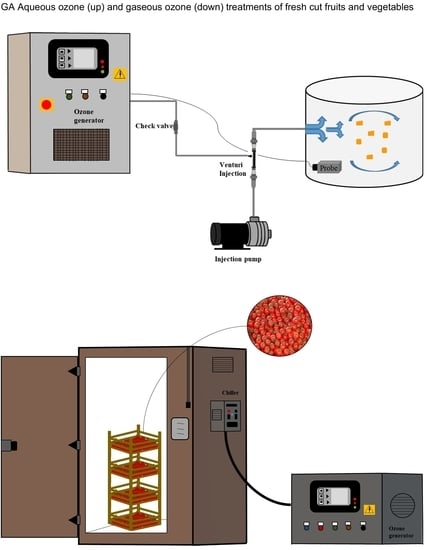
A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables
Abstract
:1. Introduction
1.1. Fresh-Cut Produce Overview
1.2. Safety and Sanitisation Practices
1.3. Technologies for Quality Preservation
1.4. Ozone Technology
2. Literature Review Aims and Objectives
3. Ozone Treatments and Fresh-Cut Fruits and Vegetables Quality
3.1. Physiological and Technological Behaviour
3.2. Chemical and Nutritional Content
3.2.1. Total Soluble Solids and Titratable Acidity
3.2.2. Fresh-Cut Browning
3.2.3. Antioxidants
3.3. Sensorial Properties
4. Microbiological Control in Fresh-Cut Fruits and Vegetables
4.1. Aqueous Ozone Treatments
4.2. Gaseous Ozone Treatments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Weng, C.-J.; Yen, G.-C. Chemopreventive Effects of Dietary Phytochemicals against Cancer Invasion and Metastasis: Phenolic Acids, Monophenol, Polyphenol, and Their Derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Toti, M.; Carboni, C.; Botondi, R. Postharvest Gaseous Ozone Treatment Enhances Quality Parameters and Delays Softening in Cantaloupe Melon during Storage at 6 °C. J. Sci. Food Agric. 2018, 98, 487–494. [Google Scholar] [CrossRef]
- Botondi, R.; Bartoloni, S.; Baccelloni, S.; Mencarelli, F. Biodegradable PLA (Polylactic Acid) Hinged Trays Keep Quality of Fresh-Cut and Cooked Spinach. J. Food Sci. Technol. 2015, 52, 5938–5945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of Polysaccharide-Based Edible Coatings Enriched with Dietary Fiber on Quality Attributes of Fresh-Cut Apples. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef] [Green Version]
- Aiyer, H.S.; Warri, A.M.; Woode, D.R.; Hilakivi-Clarke, L.; Clarke, R. Influence of Berry Polyphenols on Receptor Signaling and Cell-Death Pathways: Implications for Breast Cancer Prevention. J. Agric. Food Chem. 2012, 60, 5693–5708. [Google Scholar] [CrossRef] [Green Version]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Mencarelli, F.; Massantini, R.; Lanzarotta, L.; Botondi, R. Accurate Detection of Firmness and Colour Changes in the Packing of Table Grapes with Paper Dividers. J. Hortic. Sci. 1994, 69, 299–304. [Google Scholar] [CrossRef]
- Draft Guidance for Industry: Guide to Minimize Food Safety Hazards of Fresh-Cut Produce. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-guide-minimize-food-safety-hazards-fresh-cut-produce (accessed on 23 January 2021).
- Alzamora, S.M.; Tapia, M.S.; López-Malo, A. Minimally Processed Fruits and Vegetables: Fundamental Aspects and Applications; Aspen Food Engineering Series; Aspen Publishers: Gaithersburg, MD, USA, 2000; ISBN 978-0-8342-1672-3. [Google Scholar]
- Baselice, A.; Colantuoni, F.; Lass, D.A.; Nardone, G.; Stasi, A. Trends in EU Consumers’ Attitude towards Fresh-Cut Fruit and Vegetables. Food Qual. Prefer. 2017, 59, 87–96. [Google Scholar] [CrossRef]
- Botondi, R. Hygiene Behavior Assessment of a Hazelnut Processing Plant. Br. Food J. 2019, 121, 400–410. [Google Scholar] [CrossRef]
- EUR-Lex-32004R0852-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2004/852/oj (accessed on 29 January 2021).
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported Foodborne Outbreaks Due to Fresh Produce in the United States and European Union: Trends and Causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Althaus, D.; Kiefer, S.; Lehner, A.; Hatz, C.; Schmutz, C.; Jost, M.; Gerber, N.; Baumgartner, A.; Hächler, H.; et al. Foodborne Transmission of Listeria Monocytogenes via Ready-to-Eat Salad: A Nationwide Outbreak in Switzerland, 2013–2014. Food Control 2015, 57, 14–17. [Google Scholar] [CrossRef]
- Abadias, M.; Alegre, I.; Oliveira, M.; Altisent, R.; Viñas, I. Growth Potential of Escherichia Coli O157:H7 on Fresh-Cut Fruits (Melon and Pineapple) and Vegetables (Carrot and Escarole) Stored under Different Conditions. Food Control 2012, 27, 37–44. [Google Scholar] [CrossRef]
- Wadamori, Y.; Gooneratne, R.; Hussain, M.A. Outbreaks and Factors Influencing Microbiological Contamination of Fresh Produce. J. Sci. Food Agric. 2017, 97, 1396–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, E.; O’Beirne, D. Characterising Deterioration Patterns in Fresh-Cut Fruit Using Principal Component Analysis. II: Effects of Ripeness Stage, Seasonality, Processing and Packaging. Postharvest Biol. Technol. 2015, 100, 91–98. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.-B.; Chung, H.-S.; Moon, K.-D. Browning Control of Fresh-Cut Lettuce by Phytoncide Treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality Changes and Nutrient Retention in Fresh-Cut versus Whole Fruits during Storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef] [PubMed]
- Soliva-Fortuny, R.C.; Martín-Belloso, O. New Advances in Extending the Shelf-Life of Fresh-Cut Fruits: A Review. Trends Food Sci. Technol. 2003, 14, 341–353. [Google Scholar] [CrossRef]
- Fawell, J. Risk Assessment Case Study—Chloroform and Related Substances. Food Chem. Toxicol. 2000, 38, S91–S95. [Google Scholar] [CrossRef]
- Ali, A.; Yeoh, W.K.; Forney, C.; Siddiqui, M.W. Advances in Postharvest Technologies to Extend the Storage Life of Minimally Processed Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 2632–2649. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F. Fresh-Cut Fruit and Vegetables: Emerging Eco-friendly Techniques for Sanitation and Preserving Safety. In Postharvest Handling; Kahramanoglu, I., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Botondi, R.; Moscetti, R.; Massantini, R. A Comparative Study on the Effectiveness of Ozonated Water and Peracetic Acid in the Storability of Packaged Fresh-Cut Melon. J. Food Sci. Technol. 2016, 53, 2352–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoloni, S.; Mencarelli, F.; Forniti, R.; Botondi, R. Effect of different sanitizers on the qualitative characteristics of fresh-cut spinach under passive modified atmosphere packaging. Ind. Aliment. 2012, 51, 30–39. [Google Scholar]
- Silveira, A.C.; Aguayo, E.; Artés, F. Emerging Sanitizers and Clean Room Packaging for Improving the Microbial Quality of Fresh-Cut ‘Galia’ Melon. Food Control 2010, 21, 863–871. [Google Scholar] [CrossRef]
- Baur, S.; Klaiber, R.; Hammes, W.P.; Carle, R. Sensory and Microbiological Quality of Shredded, Packaged Iceberg Lettuce as Affected by Pre-Washing Procedures with Chlorinated and Ozonated Water. Innov. Food Sci. Emerg. Technol. 2004, 5, 45–55. [Google Scholar] [CrossRef]
- Yang, H.; Swem, B.L.; Li, Y. The Effect of PH on Inactivation of Pathogenic Bacteria on Fresh-Cut Lettuce by Dipping Treatment with Electrolyzed Water. J. Food Sci. 2003, 68, 1013–1017. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-Life of Fresh Blueberries Coated with Quinoa Protein/Chitosan/Sunflower Oil Edible Film: Quinoa Protein/Chitosan/Oil Edible Film Applied to Blueberries. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Park, S.-I.; Stan, S.D.; Daeschel, M.A.; Zhao, Y. Antifungal Coatings on Fresh Strawberries (Fragaria × Ananassa) to Control Mold Growth During Cold Storage. J. Food Sci. 2006, 70, M202–M207. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Koyuncu, F.; Dilmaçünal, T.; Güçlü, F.; Çetinbaş, M.; Kuleaşan, H. Cold Storage of Fresh-Cut “Granny Smith” Apples. Acta Hortic. 2010, 876, 307–317. [Google Scholar] [CrossRef]
- del Aguila, J.S.; Sasaki, F.F.; Heiffig, L.S.; Ortega, E.M.M.; Trevisan, M.J.; Kluge, R.A. Effect of Antioxidants in Fresh Cut Radishes during the Cold Storage. Braz. Arch. Biol. Technol. 2008, 51, 1217–1223. [Google Scholar] [CrossRef]
- Botondi, R.; Bartoloni, S.; Forniti, R.; Mencarelli, F. Quality Characterization of Minimally Processed Melon Fruit. Ind. Aliment. 2014, 53, 20–23. [Google Scholar]
- Krasaekoopt, W.; Bhandari, B. Fresh-Cut Vegetables. In Handbook of Vegetables and Vegetable Processing; Sinha, N.K., Ed.; Wiley-Blackwell: Oxford, UK, 2011; ISBN 978-0-470-95834-6. [Google Scholar]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 2, 133–158. [Google Scholar] [CrossRef]
- Gorny, J.R. A Summary of Ca and Ma Requirements and Recommendations for Fresh-Cut (Minimally Processed) Fruits and Vegetables. Acta Hortic. 2003, 600, 609–614. [Google Scholar] [CrossRef]
- Güneş, G.; Turan, D. New Technologies and Edible Coatings for Minimally Processed and Refrigerated (MPR) Fruits and Vegetables (Fresh Cuts and Freshly Squeezed Juices). In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Food Engineering Series; Springer US: Boston, MA, USA, 2017; pp. 587–617. ISBN 978-1-4939-7016-2. [Google Scholar]
- Martínez-Hernández, G.B.; Huertas, J.-P.; Navarro-Rico, J.; Gómez, P.A.; Artés, F.; Palop, A.; Artés-Hernández, F. Inactivation Kinetics of Foodborne Pathogens by UV-C Radiation and Its Subsequent Growth in Fresh-Cut Kailan-Hybrid Broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wu, A.; Qi, W.; Qi, R.; Carter, J.M.; Rasooly, R.; He, X. Effects of Electron-Beam Irradiation on Blueberries Inoculated with Escherichia Coli and Their Nutritional Quality and Shelf Life. Postharvest Biol. Technol. 2014, 95, 28–35. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed Light Treatments for Food Preservation. A Review. Food Bioprocess Technol. 2010, 3, 13. [Google Scholar] [CrossRef]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of Cold Plasma Treatment on the Functional Properties of Fresh-Cut Apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Postharvest Ozone Application for the Preservation of Fruits and Vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.-G. Microbiological Aspects of Ozone Applications in Food: A Review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Rice, R.G.; Robson, C.M.; Miller, G.W.; Hill, A.G. Uses of Ozone in Drinking Water Treatment. J. AWWA 1981, 73, 44–57. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Graham, D.M. Use of Ozone for Food Processing. Food Technol. USA 1997, 51, 72–75. [Google Scholar]
- Scientific Opinion on the Risk Posed by Pathogens in Food of Non-Animal Origin. Part 2 (Salmonella and Norovirus in Leafy Greens Eaten Raw as Salads). EFSA J. 2014, 12, 3600. [Google Scholar] [CrossRef] [Green Version]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of Ozone in the Food Industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Carletti, L.; Botondi, R.; Moscetti, R.; Stella, E.; Monarca, D.; Cecchini, M.; Massantini, R. Use of Ozone in Sanitation and Storage of Fresh Fruits and Vegetables. J. Food Agric. Environ. 2013, 11, 585–589. [Google Scholar]
- Pascual, A.; Llorca, I.; Canut, A. Use of Ozone in Food Industries for Reducing the Environmental Impact of Cleaning and Disinfection Activities. Trends Food Sci. Technol. 2007, 18, 529–535. [Google Scholar] [CrossRef]
- Cullen, P.J.; Valdramidis, V.P.; Tiwari, B.K.; Patil, S.; Bourke, P.; O’Donnell, C.P. Ozone Processing for Food Preservation: An Overview on Fruit Juice Treatments. Ozone Sci. Eng. 2010, 32, 166–179. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Saeed, P.A. Sanitization Potential of Ozone and Its Role in Postharvest Quality Management of Fruits and Vegetables. Food Eng. Rev. 2020, 12, 48–67. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Ozone Applications in Fruit and Vegetable Processing. Food Rev. Int. 2007, 23, 91–106. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Santos-Pedro, D.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of Aqueous Ozone, Blanching and Combined Treatments on Microbial Load of Red Bell Peppers, Strawberries and Watercress. J. Food Eng. 2011, 105, 277–282. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone: An Emerging Technology for the Seafood Industry. Braz. Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Vettraino, A.; Vinciguerra, V.; Pacini, G.; Forniti, R.; Goffi, V.; Botondi, R. Gaseous Ozone as a Suitable Solution for Postharvest Chestnut Storage: Evaluation of Quality Parameter Trends. Food Bioprocess Technol. 2019, 13, 187–193. [Google Scholar] [CrossRef]
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of Antioxidant System to Postharvest Ozone Treatment in ‘Soreli’ Kiwifruit. J. Sci. Food Agric. 2020, 100, 961–968. [Google Scholar] [CrossRef]
- Goffi, V.; Zampella, L.; Forniti, R.; Petriccione, M.; Botondi, R. Effects of Ozone Postharvest Treatment on Physicochemical and Qualitative Traits of Actinidia Chinensis ‘Soreli’ during Cold Storage. J. Sci. Food Agric. 2019, 99, 5654–5661. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Zhang, Y.; Zhao, Q.; Hu, W. Effects of Aqueous Ozone Treatment on Microbial Growth, Quality, and Pesticide Residue of Fresh-cut Cabbage. Food Sci. Nutr. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of Ozone and Chlorine Washes and Subsequent Cold Storage on Microbiological Quality and Shelf Life of Fresh Parsley Leaves. LWT 2020, 127, 109421. [Google Scholar] [CrossRef]
- Nie, M.; Wu, C.; Xiao, Y.; Song, J.; Zhang, Z.; Li, D.; Liu, C. Efficacy of Aqueous Ozone Combined with Sodium Metasilicate on Microbial Load Reduction of Fresh-Cut Cabbage. Int. J. Food Prop. 2020, 23, 2065–2076. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Zhao, Q.; Liu, S.; Hu, W. Effects of Ozonated Water on Microbial Growth, Quality Retention and Pesticide Residue Removal of Fresh-Cut Onions. Ozone Sci. Eng. 2020, 42, 399–407. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Sun, Y.; Li, C.; Li, Y.; Zhang, Q.; Wu, Z. Reduction of Escherichia Coli O157:H7 and Naturally Present Microbes on Fresh-Cut Lettuce Using Lactic Acid and Aqueous Ozone. RSC Adv. 2019, 9, 22636–22643. [Google Scholar] [CrossRef] [Green Version]
- Ummat, V.; Singh, A.K.; Sidhu, G.K. Effect of Aqueous Ozone on Quality and Shelf Life of Shredded Green Bell Pepper (Capsicum Annuum). J. Food Process. Preserv. 2018, 42, jfpp.13718. [Google Scholar] [CrossRef]
- Yucel Sengun, I.; Kendirci, P. Potential of Ozonated Water at Different Temperatures to Improve Safety and Shelf-Life of Fresh Cut Lettuce. Ozone Sci. Eng. 2018, 40, 216–227. [Google Scholar] [CrossRef]
- Papachristodoulou, M.; Koukounaras, A.; Siomos, A.S.; Liakou, A.; Gerasopoulos, D. The Effects of Ozonated Water on the Microbial Counts and the Shelf Life Attributes of Fresh-Cut Spinach. J. Food Process. Preserv. 2018, 42, e13404. [Google Scholar] [CrossRef]
- Pongprasert, N.; Jitareerat, P.; Srilaong, V. A Novel Technique Using Ozone Micro Bubbles to Control Microbial Contamination and Browning of Fresh-Cut Lettuce. Acta Hortic. 2016, 1120, 177–181. [Google Scholar] [CrossRef]
- Renumarn, P.; Srilaong, V.; Uthairatanakij, A.; Kanlayanarat, S.; Jitareerat, P. The Effects of Immersion Methods and Concentration of Ozonated Water on the Microbial Counts and the Quality and Sensory Attributes of Fresh-Cut Broccoli. Int. Food Res. J. 2014, 21, 533–539. [Google Scholar]
- Beaulieu, J.C. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 2006, 41, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, E.; Escalona, V.; Silveira, A.C.; Artés, F. Quality of Tomato Slices Disinfected with Ozonated Water. Food Sci. Technol. Int. 2014, 20, 227–235. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Baldwin, E.A. Flavor and aroma of fresh-cut fruits and vegetables. In Fresh Cut Fruits and Vegetables: Science, Technology and Market; Lamikanra, O., Ed.; CRC Press: Abingdon, UK, 2002; pp. 391–425. ISBN 9781587160301. [Google Scholar]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of Ozone Efficacy on the Reduction of Microbial Population of Fresh Cut Lettuce (Lactuca Sativa) and Green Bell Pepper (Capsicum Annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Renumarn, P.; Srilaong, V.; Uthairatanakij, A.; Kanlayanarat, S.; Jitareerat, P. Contact Time of Ozonated Water on Microbial Populations and Quality Changes of Fresh-Cut Broccoli Florets. Acta Hortic. 2013, 177–183. [Google Scholar] [CrossRef]
- Calder, B.L.; Skonberg, D.I.; Davis-Dentici, K.; Hughes, B.H.; Bolton, J.C. The Effectiveness of Ozone and Acidulant Treatments in Extending the Refrigerated Shelf Life of Fresh-Cut Potatoes. J. Food Sci. 2011, 76, S492–S498. [Google Scholar] [CrossRef]
- Das, B.K.; Kim, J.G.; Choi, J.W. Efficacy of Different Washing Solutions and Contact Times on the Microbial Quality and Safety of Fresh-Cut Paprika. Food Sci. Technol. Int. 2011, 17, 471–479. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Raju, P.S.; Ravi, N.; Singh, A.; Bawa, A.S. Effectiveness of Ozone in Combination with Controlled Atmosphere on Quality Characteristics Including Lignification of Carrot Sticks. J. Food Eng. 2011, 102, 43–48. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. The Effects of Gaseous Ozone and Chlorine on Quality and Shelf Life of Minimally Processed Red Pepper. Acta Hortic. 2010, 877, 583–589. [Google Scholar] [CrossRef]
- Das, B.K.; Kim, J.-G. Microbial Quality and Safety of Fresh-Cut Broccoli with Different Sanitizers and Contact Times. J. Microbiol. Biotechnol. 2010, 20, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ölmez, H.; Akbas, M.Y. Optimization of Ozone Treatment of Fresh-Cut Green Leaf Lettuce. J. Food Eng. 2009, 90, 487–494. [Google Scholar] [CrossRef]
- Selma, M.V.; Allende, A.; Lòpez-Galvèz, F.; Conesa, M.A.; Gil, M.I. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Akbas, M.Y.; Ölmez, H. Effectiveness of Organic Acid, Ozonated Water and Chlorine Dippings on Microbial Reduction and Storage Quality of Fresh-Cut Iceberg Lettuce. J. Sci. Food Agric. 2007, 87, 2609–2616. [Google Scholar] [CrossRef]
- Hassenberg, K.; Idler, C.; Molloy, E.; Geyer, M.; Plöchl, M.; Barnes, J. Use of Ozone in a Lettuce-Washing Process: An Industrial Trial. J. Sci. Food Agric. 2007, 87, 914–919. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and Measuring the Quality of Fresh-Cut Fruit and Vegetables: A Review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Koseki, S.; Isobe, S. Effect of Ozonated Water Treatment on Microbial Control and on Browning of Iceberg Lettuce (Lactuca Sativa L.). J. Food Prot. 2006, 69, 154–160. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation of Fresh-Cut Celery by Treatment of Ozonated Water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Beltrán, D.; Selma, M.V.; Marín, A.; Gil, M.I. Ozonated Water Extends the Shelf Life of Fresh-Cut Lettuce. J. Agric. Food Chem. 2005, 53, 5654–5663. [Google Scholar] [CrossRef]
- Beltrán, D.; Selma, M.V.; Tudela, J.A.; Gil, M.I. Effect of Different Sanitizers on Microbial and Sensory Quality of Fresh-Cut Potato Strips Stored under Modified Atmosphere or Vacuum Packaging. Postharvest Biol. Technol. 2005, 37, 37–46. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Zhang, Y.; Jiang, A.; Hu, W. Aqueous ozone treatment inhibited degradation of cellwall polysaccharides in fresh-cut apple during cold storage. Innov. Food Sci. Emerg. Technol. 2021, 67, 1466–8564. [Google Scholar] [CrossRef]
- Silveira, A.C.; Oyarzún, D.; Escalona, V. Oxidative Enzymes and Functional Quality of Minimally Processed Grape Berries Sanitised with Ozonated Water. Int. J. Food Sci. Technol. 2018, 53, 1371–1380. [Google Scholar] [CrossRef]
- Liu, C.; Ma, T.; Hu, W.; Tian, M.; Sun, L. Effects of Aqueous Ozone Treatments on Microbial Load Reduction and Shelf Life Extension of Fresh-Cut Apple. Int. J. Food Sci. Technol. 2016, 51, 1099–1109. [Google Scholar] [CrossRef]
- Nur Aida, M.P.; Hairiyah, M.; Wan Mohd Reza, W.H.; Nur Ilida, M. Effect of Ozonated Water Wash on Quality of Fresh-Cut “Josapine” Pineapple During Storage. Acta Hortic. 2011, 902, 487–492. [Google Scholar] [CrossRef]
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A.A. Ozone-Induced Changes of Antioxidant Capacity of Fresh-Cut Tropical Fruits. Innov. Food Sci. Emerg. Technol. 2010, 11, 666–671. [Google Scholar] [CrossRef]
- Gutiérrez, D.R.; Chaves, A.R.; Rodríguez, S.D.C. Use of UV-C and Gaseous Ozone as Sanitizing Agents for Keeping the Quality of Fresh-Cut Rocket (Eruca Sativa Mill): UV-C and Ozone Keeping the Quality of Fresh- Cut Rocket. J. Food Process. Preserv. 2017, 41, e12968. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wang, J.; Hu, H.; Cui, H. Combined Effect of Ozone Treatment and Modified Atmosphere Packaging on Antioxidant Defense System of Fresh-Cut Green Peppers. J. Food Process. Preserv. 2016, 40, 1145–1150. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of Ozone Treatments on Microbial Quality and Some Chemical Properties of Lettuce, Spinach, and Parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.H.; Artes, F. Effect of Cyclic Exposure to Ozone Gas on Physicochemical, Sensorial and Microbial Quality of Whole and Sliced Tomatoes. Postharvest Biol. Technol. 2006, 39, 169–177. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C.F. Effects of Ozone on Major Antioxidants and Microbial Populations of Fresh-Cut Papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Dilmaçünal, T.; Erbaş, D.; Koyuncu, M.A.; Onursal, C.E.; Kuleaşan, H. Efficacy of Some Antimicrobial Treatments Compared to Sodium Hypochlorite on Physical, Physiological and Microbial Quality of Fresh-Cut Melons (Cucumis Melo L. Var. Inodorus). LWT Food Sci. Technol. 2014, 59, 1146–1151. [Google Scholar] [CrossRef]
- Selma, M.V.; Ibáñez, A.M.; Cantwell, M.; Suslow, T. Reduction by Gaseous Ozone of Salmonella and Microbial Flora Associated with Fresh-Cut Cantaloupe. Food Microbiol. 2008, 25, 558–565. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Mencarelli, F.; Garosi, F.; Botondi, R.; Tonutti, P. Postharvest Physiology of Peach and Nectarine Slices. Acta Hortic. 1998, 465, 463–470. [Google Scholar] [CrossRef]
- Sanchís, E.; Ghidelli, C.; Sheth, C.C.; Mateos, M.; Palou, L.; Pérez-Gago, M.B. Integration of Antimicrobial Pectin-Based Edible Coating and Active Modified Atmosphere Packaging to Preserve the Quality and Microbial Safety of Fresh-Cut Persimmon (Diospyros Kaki Thunb. Cv. Rojo Brillante): Methods to Preserve the Quality and Safety of Cut Persimmon. J. Sci. Food Agric. 2017, 97, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Toivonen, P.M.A.; Delaquis, P.J. Effect of Wash Water Temperature and Chlorination on Phenolic Metabolism and Browning of Stored Iceberg Lettuce Photosynthetic and Vascular Tissues. J. Agric. Food Chem. 2002, 50, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; Cardarelli, M.; Mencarelli, F. The Role of Ethylene in Regulating Cell Wall-Degrading Enzyme Activity Using Antisense Acc-Oxidase in Cantaloupe Melons. Acta Hortic. 2000, 510, 471–478. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Hadfield, K.A.; Labavitch, J.M.; Bennett, A.B. Temporal Sequence of Cell Wall Disassembly in Rapidly Ripening Melon Fruit. Plant Physiol. 1998, 117, 345–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-cut fruits and vegetables: Quality issues and safety concerns. In Fresh-Cut Fruits and Vegetables, 1st ed.; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. ISBN 978-0-12-816184-5. [Google Scholar]
- Rico, D.; Martín-Diana, A.B.; Frías, J.M.; Henehan, G.T.; Barry-Ryan, C. Effect of Ozone and Calcium Lactate Treatments on Browning and Texture Properties of Fresh-Cut Lettuce. J. Sci. Food Agric. 2006, 86, 2179–2188. [Google Scholar] [CrossRef]
- Roy, S.S.; Taylor, T.A.; Kramer, H.L. Textural and Ultrastructural Changes in Carrot Tissue as Affected by Blanching and Freezing. J. Food Sci. 2001, 66, 176–180. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Pyatkovskyy, T.; Mohamed, H.M.; Yousef, A.E.; Sastry, S.K. Physics of Fresh Produce Safety: Role of Diffusion and Tissue Reaction in Sanitization of Leafy Green Vegetables with Liquid and Gaseous Ozone-Based Sanitizers. J. Food Prot. 2015, 78, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Kaur, B.; Gupta, K.; Kumar, S. Studies on Refrigerated Storage of Minimally Processed Papaya (Carica Papaya L.). Agric. Eng. Int. CIGR J. 2013, 15, 275–280. [Google Scholar]
- Amaral, R.D.; Bachelli, M.L.; Zerbinati, M.; Benedetti, B.C. Effectiveness of Different Concentrations of Ozonated Water in the Sanitization of Fresh-Cut Green Pepper. CIGR Proc. 2009, 14, 131–135. [Google Scholar]
- Eissa, H.A.; Fadel, H.H.M.; Ibrahim, G.E.; Hassan, I.M.; Elrashid, A.A. Thiol Containing Compounds as Controlling Agents of Enzymatic Browning in Some Apple Products. Food Res. Int. 2006, 39, 855–863. [Google Scholar] [CrossRef]
- Patil, S.; Torres, B.; Tiwari, B.K.; Wijngaard, H.H.; Bourke, P.; Cullen, P.J.; O’ Donnell, C.P.; Valdramidis, V.P. Safety and Quality Assessment during the Ozonation of Cloudy Apple Juice. J. Food Sci. 2010, 75, M437–M443. [Google Scholar] [CrossRef]
- Sachadyn-Król, M.; Agriopoulou, S. Ozonation as a Method of Abiotic Elicitation Improving the Health-Promoting Properties of Plant Products—A Review. Molecules 2020, 25, 2416. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Harker, F.R.; Gunson, F.A.; Jaeger, S.R. The case for fruit quality: An interpretive review of consumer attitudes, and preferences for apples. Postharvest Biol. Technol. 2003, 28, 333–347. [Google Scholar] [CrossRef]
- Sothornvit, R. Effect of ozonated and chlorinated water on quality of fresh-cut cauliflower and basil. Acta Hortic. 2008, 858, 319–324. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological Control of Postharvest Diseases of Fruits and Vegetables by Microbial Antagonists: A Review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Food. Microbiologia Degli Alimenti; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-88-470-0785-7. [Google Scholar]
- Potter, A.; Murray, J.; Lawson, B.; Graham, S. Trends in Product Recalls within the Agri-Food Industry: Empirical Evidence from the USA, UK and the Republic of Ireland. Trends Food Sci. Technol. 2012, 28, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C.D. Understanding the Routes of Contamination of Ready-to-Eat Vegetables in the Middle East. Food Control 2016, 62, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Miller, F.A.; Ramos, B.; Gil, M.M.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Influence of PH, Type of Acid and Recovery Media on the Thermal Inactivation of Listeria Innocua. Int. J. Food Microbiol. 2009, 133, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.E. Natural antimicrobials from plants. In New Methods of Food Preservation; Gould, G.W., Ed.; Springer US: Boston, MA, USA, 1995; pp. 58–89. ISBN 978-1-4615-2105-1. [Google Scholar]
- Ayala-Zavala, J.F.; Oms-Oliu, G.; Odriozola-Serrano, I.; González-Aguilar, G.A.; Álvarez-Parrilla, E.; Martín-Belloso, O. Bio-Preservation of Fresh-Cut Tomatoes Using Natural Antimicrobials. Eur. Food Res. Technol. 2008, 226, 1047–1055. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent Developments in Novel Shelf Life Extension Technologies of Fresh-Cut Fruits and Vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Glowacz, M.; Rees, D. The Practicality of Using Ozone with Fruit and Vegetables. J. Sci. Food Agric. 2016, 96, 4637–4643. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Ibáñez, A.M.; Allende, A.; Cantwell, M.; Suslow, T. Effect of Gaseous Ozone and Hot Water on Microbial and Sensory Quality of Cantaloupe and Potential Transference of Escherichia Coli O157:H7 during Cutting. Food Microbiol. 2008, 25, 162–168. [Google Scholar] [CrossRef]
- Gil Muñoz, M.I.; Allende, A.; Selma, M.V. Treatments to Assure Safety of Fresh-Cut Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-7123-8. [Google Scholar]
- Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro–Nanobubble Technology and Water-Related Application. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]

| Vegetables | Treatment | Qualitative Results | Microbiological Results | References |
|---|---|---|---|---|
| Cabbage | Aqueous ozone (1.4 mg L−1) for 1, 5 and 10 min | The aqueous ozone (AO) treatment stimulated initial respiratory metabolism, reducing ethylene production and improved the overall quality of fresh-cut cabbage, compared with that of the control. The effect of treatment for 5 min on the removal of trichlorfon, chlorpyrifos, methomyl, dichlorvos and omethoate was greater than that of the control. | The growth rates of aerobic bacteria, coliforms and yeasts were significantly inhibited by ozonated water; longer treatment time (10 min) showed the greatest inactivation of bacteria, coliforms and molds. | Liu et al., 2021 [63] |
| Parsley leaves | Aqueous ozone 12.0 mg L−1 compared with chlorine 100 mg L−1 (5 min) | Chlorophylls, ascorbic acid, total phenolic contents and antioxidant activity were not adversely affected by washing treatments tested (aqueous ozone and chlorine). | Reduction in E. coli counts were observed in the samples, while L. innocua counts were stable during storage. Maximum reductions in the counts of L.innocua and E.coli were obtained with chlorinated water treatment. However, reduction in L. innocua count obtained with chlorinated water was not significantly higher than that obtained with ozonated water. Therefore, ozone could be considered as an alternative to chlorine for L. innocua | Karaca and Velioglu, 2020 [64] |
| Cabbage | Aqueous ozone 2 ppm combined with 0.4% sodium metasilicate for 2 min | This combination treatment had no negative effects on sensory characteristics (appearance and off-odor), color and the contents of ascorbic acid, total phenols and carotenoids. | This combination treatment achieved acceptable microbial quality and reduced E. coli counts by 3.33 logs units compared with the control samples after 12 d of storage. | Nie et al., 2020 [65] |
| Onion | Aqueous ozone 1.4 mg L−1 for 1, 3 and 5 min | AO treatment for 1 min significantly reduced the weight loss of fresh-cut onions during a longer storage time (8–14 days). All AO treatments reduced the respiration rate and the softening of fresh-cut onions. AO treatment for 5 min significantly reduced the residual levels of five tested pesticides (dimethyl dichlorovinyl phosphate, cypermethrin, chlorpyrifos, methomyl and omethoate) compared with water treatment. | The results showed an inhibition of the growth of aerobic bacteria, coliforms and yeasts during storage, with the AO treatment for 5 min allowing the lowest growth rates. | Chen et al., 2020 [66] |
| Lettuce | Aqueous ozone 1 mg L−1 (30 s) or 2 mg L−1 (30 s) with 1% lactic acid (90 s) | Quality analysis (color, sensory qualities, electrolyte leakage, polyphenolic content and weight loss) showed that lactic acid (LA) + AO did not cause additional quality loss compared with tap water treatment. | Microbial analysis showed that LA plus AO led to the greatest reductions in microbes (Escherichia coli O157:H7, aerobic mesophilic counts, aerobic psychrophilic counts, molds and yeasts) during storage (0–5 days at 5 °C) | Wang et al., 2019 [67] |
| Green bell pepper | Aqueous ozone 1–3 mg L−1 1–5 min | The exposure to ozone treatments above 2.4 mg L−1 for higher durations showed better retention of other quality parameters such as ascorbic acid, firmness, color and overall acceptability. | The exposure to ozone treatments above 2.4 mg L−1 for higher durations significantly reduced the microbial load. | Ummat et al., 2018 [68] |
| Lettuce | Aqueous ozone 2 mg L−1 at different temperatures (4 and 15 °C) | During storage period at 4 °C for 14 days, the highest quality was observed from the samples treated with cold ozonated water. Any effect on color properties. | Cold ozone treatment (4 °C) significantly reduced the natural background microflora of lettuce. Salmonella enterica serovar Typhimurium and Escherichia coli inoculated on lettuce samples were insignificantly influenced by the temperature of water. | Sengun et al., 2018 [69] |
| Spinach | Aqueous ozone 0.4, 0.8 and 1.2 mg L−1 for 1, 15 or 30 s | Ozonated water (0.8 mg L−1 for 30 s) before packaging reduced yellowing and maintained compositional characteristics of the fresh-cut spinach leaves, ensuring a shelf life extension of 3 days. | The microbiological analyses indicated the ability of ozonated water to decrease the Gram-negative and Enterobacteriaceae sp. load only during the first 5 days of storage (0.8 mg L−1 for 30 s). | Papachristodoulou et al., 2018 [70] |
| Lettuce | Aqueous ozone 0.5 ppm for 5 min | The amount of phenolic compounds of the lettuce was reduced by AO. Washing with AO also inhibited polyphenol oxidase (PPO) activity for up to six days of storage at 4 °C, which is correlated with a lower amount of quinone content during storage. An inhibitory effect of browning enzyme and substrate resulted in a lower browning symptom. | For total bacteria counts, coliform counts and yeast and mold, ozone microbubbles (O3−MBs) treatment resulted into a 1–2 log reduction, which was similar to the result achieved by 100 mg L−1 chlorinated water. | Pongprasert et al., 2016 [71] |
| Broccoli | Aqueous ozone 0.56 ppm ozone for 5 min and 1.60 ppm for 3 min) | This treatment did not have negative effects on color (lightness, a*, b* and hue angle values), chlorophyll content or sensory attributes (overall visual quality, visible color and odor). | All treatments reduced the amount of microbes compared with the initial microbial loads of unwashed fresh-cut broccoli. Treatment with 1.60 ppm was the most effective treatment with regard to reducing aerobic bacteria, coliforms and yeasts and molds. | Renumarn et al., 2014 [72] |
| Lettuce, spinach and parsley | Aqueous ozone 12 mg L−1 Gaseous ozone 950 μL L−1, 20 min | AO does not affect chemical characteristics of the vegetables (chlorophyll a, chlorophyll b, ascorbic acid and total phenolic contents and antioxidant activity). Gaseous ozone (GO) caused significant losses in important bioactive compounds of parsley. Ascorbic acid and total phenolic contents and antioxidant activity in ozone-treated samples were 40.1, 14.4 and 41.0%, respectively, less than the control samples. | Aqueous ozone: chlorine and ozone washes resulted in average log units reductions of 2.9 and 2.0 for E. coli in the vegetables tested, respectively, while the efficiency of ozone (2.2 log) was very close to that of chlorine (2.3 log) on L. innocua. GO treatment resulting in 1.0–1.5 log reductions in the numbers of E. coli and L. innocua in parsley. | Karaca and Velioglu, 2014 [73] |
| Tomato slices | Aqueous ozone 0.4 mg L−1 for 1, 3 and 5 min | Ozonated water treatment of 3 min achieved the best firmness retention and reduced the consumption of fructose and glucose. The use of ozonated water did not affect the total acidity, pH, total solid soluble, organic acid as ascorbic, fumaric or succinic acid and the sensorial parameters. | Ozonated water treatment of 3 min achieved the best microbial quality (mesophilic, psychrotrophic and yeast load) | Aguayo et al., 2014 [74] |
| Bell peppers | Aqueous ozone 1 μL L−1 Gaseous ozone 0.7 μL L−1 | In all the experiments, O2 continuously decreased and CO2 concentration increased. The pH value increased, and a significant softening was observed in all the fruits. By day 14, L values decreased in all the fruits, with the greatest changes found in the chlorinated samples (approximately 12 units). The aqueous solutions, ozonated (1 μL L−1) and chlorine (200 μL L−1) water showed greater changes in the quality attributes with increasing washing times. The GO treatment had no effect on the physicochemical attributes studied. | The exposure for three min to gaseous ozone reduced the mesophiles, psychrotrophes and fungal populations of the fresh-cut peppers in 2.5, 3.3 and 1.8 log units, respectively, compared to ozone treatment in water. | Horvitz et al., 2014 [75] |
| Lettuce and green bell pepper | Aqueous ozone 0.5 mg L−1 | This vegetables dipped in chlorinated water (20 ppm) resulted in a 1 log decrease in the total microbial count in the first 15 min. The immersion of vegetables in water pre-saturated with ozone did not make any difference because the total microbial count decreased approximately 0.5 log for the same time. Sanitation treatments were most effective when vegetables were dipped in continuously ozonated (0.5 mg L−1) water, leading to about 2 log of microbial load decrease in the first 15 min and 3.5 log after 30 min of exposure. | Alexopoulos et al., 2013 [76] | |
| Broccoli florets | Aqueous ozone 0.56, 1.00 and 1.50 ppm | Ozonated water treatments impacted fresh-cut broccoli quality by decreasing the chlorophyll contents, L* value and hue angle. The visual quality and visual color evaluated by the sensory panel in both ozonated water and non-ozonated water treatments were not significantly different. | Application of ozonated water for 15 min significantly reduced coliforms, total bacteria and yeast and mold counts by 1.20, 2.50 and 1.80 log10 CFU.g−1, respectively, when compared with that of the control. | Renumarn et al., 2013 [77] |
| Spinach | Aqueous ozone 3 ppm for 4 min | The treatments with ozonated water (3 ppm for 4 min) and water tsunami solution (300 ppm for 4 min) allowed the color to be maintained during storage. Moreover, results suggest that sanitization with ozonated water causes an initial dejection of ethylene production in addition to a phenolic content decrease coupled with a lower antioxidant activity. | Bartoloni et al., 2012 [27] | |
| Potatoes slices | Aqueous ozone 2 ppm | Acidulant dip treatments with aqueous ozone (2 ppm) had significantly higher L-values and lower a-values. NatureSeal (NS) and sodium acid sulfate (SAS) were the most effective acidulant treatments in reducing browning (higher L-values, lower a-values and browning index values) regardless of ozone treatment. Ozone did not appear to have any effect on polyphenol oxidase (PPO) activity. NS and SAS also had lower PPO activity compared to other treatments. | Ozone did not appear to have any effect on aerobic plate counts (APCs). NS and SAS also had lower PPO activity compared to other treatments and significantly lower APCs. | Calder et al., 2011 [78] |
| Paprika | Aqueous ozone 4 mg L−1 for 90 and 180 s | All washing solutions (tap water, chlorinated water 100 mg L−1 and pH 6.5–7, electrolyzed water pH 7.2 and ozonized water 4 mg L−1) showed insignificant differences in gas composition, and no off-odor was detected. Longer contact time resulted in slightly lower hue angle value than a short one for all washing solutions. Samples washed with ozone washings showed lower electrolyte leakage than other washing solutions. | Samples washed for longer contact time except those washed in ozonized water showed increased microbial numbers during storage. | Das and Kim, 2011 [79] |
| Red bell peppers, strawberries and watercress | Aqueous ozone (0.3 e 2 mg L−1) for 1, 2 and 3 min | The highest microbial reductions were obtained for the highest concentration with the highest treatment time (3 min). Listeria innocua in fresh-cut peppers, total mesophilic bacteria in fresh-cut strawberries and total coliforms in fresh-cut watercress were tested, resulting in a microbial load reduction of 2.8, 2.3 and 1.7 log cycles, respectively, compared to the control. | Alexandre et al., 2011 [58] | |
| Carrot sticks | Aqueous ozone 200 mg h−1 for 10 min | The maximum decrease in respiration and ethylene emission rates were obtained by the combination of CA with ozone. Significant reduction in ascorbic acid, carotenoids and oxidative enzymes such as polyphenol oxidase (PPO) and peroxidase (POD) were observed due to ozonation and CA storage. The control of lignification by ozone in synergy with CA was characterized by a decrease in L values. | The ozonation in combination with CA have a positive role in controlling microbial spoilage. | Chauhan et al., 2011 [80] |
| Red pepper | Aqueous ozone 0.7 ppm and chlorinated water (200 ppm) for 1, 3 and 5 min | Weight loss was negligible. O2 concentration decreased and CO2 levels increased continuously, with no differences between treatments for the ozonated samples. With chlorine, changes in the gas composition were more accentuated. AO treatments does not affect physicochemical parameters. Color was not affected by O3 immediately after the treatment, and no surface discoloration was observed. | Ozonated water reduced the yeasts, molds, aerobic mesophilic and psychrotrophic bacteria counts. On the other hand, chlorine was not effective to reduce aerobic mesophilic bacteria counts (for yeasts and molds and psychrotrophic bacteria, the best results were obtained when washing for 1 min). | Horvitz and Cantalejo, 2010 [81] |
| Broccoli | Aqueous ozone 2 μL L−1 for 90 s and 180 s | No significant differences were observed in gas composition and color parameters among different sanitizers (100 μL L−1 chlorinated water, electrolyzed water containing 100 μL L−1 free chlorine, 2 μL L−1 ozonated water) with contact times. No off-odor was detected during the storage. | A longer contact time was not effective in reducing microbial population, except with O3 washing. O3 with 90 s was not very effective in reducing microbial population compared with Cl or EW. However, samples washed with O3 for 180 s observed the lowest numbers of total aerobic and coliform plate counts. | Das and Kim, 2010 [82] |
| Green leaf lettuce | Aqueous ozone 2 ppm for 2 min | Ozone treatment showed no significant effect on ascorbic acid and d β-carotene contents and was found to be better than the chlorine and organic acid treatments in maintaining the sensory quality, compared with chlorinated water (100 ppm) and organic acid treatments. | No significant difference was observed between these three treatments in reducing the microbial load and controlling it during cold storage. | Ölmez and Akbas, 2009 [83] |
| Onion, escarole, carrot and spinach | Aqueous ozone 10, 20 and 80 mg min−1 | Turbidity of wash water was reduced significantly by O3 and O3−UV treatments, while UV treatment did not affect the physicochemical quality of the water. | UV and O3−UV were effective disinfection treatments on vegetable wash water, with a maximum microbial reduction with O3−UV. However, maximum total microbial reductions were achieved by UV and O3 treatments, lower than by O3−UV treatment. | Selma et al., 2008 [84] |
| Iceberg lettuce | Aqueous ozone 4 mg L−1 | No significant changes were observed in the texture and moisture content of lettuce samples dipped in chlorine, organic acids and ozonated water during storage. Color, β-carotene and ascorbic acid values of fresh-cut iceberg lettuce did not change significantly. | Organic acid dippings resulted in lower mesophilic and psychrotrophic counts than ozonated water and chlorine dippings. | Akbas and Olmez, 2007 [85] |
| Lettuce | Aqueous ozone 3.6 ppm | Through the addition of ozone to the wash water, the quality of lettuce during storage time was unaffected compared with water-washed lettuce. | Through the addition of ozone to the wash water, there was only a limited observed decrease in populations of microorganisms, compared with water-washed lettuce. | Hassenberg et al., 2007 [86] |
| Lettuce | Aqueous ozone 1 mg L−1 | The use of ozone produced a significantly higher oxygen decline than the use of CLac (calcium lactate,15 g L−1). At the end of storage, CLac (alone or combined with ozone) samples had higher oxygen content (∼9%) than ozone samples (∼6%). Significant reductions in POD, PPO and enzymatic activity (polyphenol oxidase, peroxidase and pectin methylesterase activity) were observed in ozone samples. The reduction in pectin methylesterase activity has negatively affected textural properties. | Rico et al., 2007 [87] | |
| Iceberg lettuce | Aqueous ozone 3, 5 and 10 ppm for 5 min | Ozonated water treatment increased the phenylalanine ammonia lyase (PAL) activity, compared with the water wash treatment, but the concentration of ozone did not affect PAL activity. Treatment with 3 or 5 ppm ozonated water resulted in more rapid changes in the a* value than after the water treatment. The ascorbic acid content of the lettuce was not affected by these treatments. | The native bacterial population on the lettuce declined in response to a rise in ozone concentration. However, there was no further bacterial reduction above 5 ppm ozone. | Koseki and Isobe, 2006 [88] |
| Celery | Aqueous ozone 0.03, 0.08, 0.18 ppm for 2, 6, 10 min, respectively | The polyphenoloxidase (PPO) activity and respiration rate was significant inhibited by treatment of ozonated water, and sensory quality of fresh-cut celery treated with ozonated water was better than that non-treated (at 0.18 ppm). There is no significant difference between ascorbic acid and total sugar of fresh-cut celery treated with ozonated water and non-treated. | Significant reduction of 1.7 log cycles of total microbial counts in fresh-cut celery treated with ozonated water at 0.18 ppm. | Zhang et al., 2005 [89] |
| Lettuce | Aqueous ozone 10, 20 and 10 mg L−1 min, activated by ultraviolet C (UV-C) light, in air or active modified atmosphere packaging (MAP) | Despite its strong oxidizing activity, ozonated water did not stimulate the respiratory activity of fresh-cut lettuce. Ozonated water maintained the initial visual appearance of fresh-cut lettuce and controlled browning during storage in air. | Initially, ozonated water and chlorine reduced the total mesophilic population by 1.6 and 2.1 log, respectively, when compared with water. Active MAP was effective in controlling total microbial growth, in relation to samples stored in air and caused a reduction in coliforms on sanitized samples compared with water-washed samples. The most efficient treatments were ozone 20 and ozone 10 activated by UV-C, which were as effective as chlorine. | Beltrán et al., 2005 [90] |
| Potato strips | Aqueous ozone 20 mg L−1 min | Under MAP, only sodium sulfite prevented browning, although it conferred off-odors, compared with other treatments (water, sodium hypochlorite, Tsunami, ozone and the combination of ozone–Tsunami). After 14 days of storage, there was no evidence of browning in fresh-cut potatoes dipped in ozonated water or ozone–Tsunami, and these treatments maintained initial texture and aroma, compared with other treatments. | The use of ozonated water alone was not effective in reducing total microbial populations. Ozone–Tsunami resulted in the most effective treatment to control microbial growth. | Beltrán et al., 2005 [91] |
| Fruits | Treatment | Qualitative Results | Microbiological Results | References |
|---|---|---|---|---|
| Apple | Aqueous ozone 1.4 mg L−1 for 5 min | Water-soluble pectin content increased more slowly, while protopectin content and cellulose content decreased at a lower rate in AO-treated fresh-cut apple compared with the control. AO treatment promoted increased pectin methylesterase activity and distinctly inhibited β-galactosidase and α-arabinofuranosidase activities during storage. Polygalacturonase activity was not affected by AO treatment. | Liu et al., 2020 [92] | |
| Grapes | Aqueous ozone 2, 4, 6, 8 mg L−1 | Ozonated water stimulated the respiration rate, especially after 5 days of storage, and increased superoxide dismutase and catalase activity compared to NaOCl (100 mg L−1) sanitized grapes. Total polyphenol content was 23–50% higher in Thompson Seedless (TS) and 18.5–28% higher in and Black (BS) samples sanitized with ozonated water. Twofold higher total antioxidant capacity (TAC) was registered in TS at all of the evaluated O3 doses while the doses of 6 and 8 mg L−1 increased TAC by 19–30% in BS. The use of ozonated water as a sanitizing method, especially at 6 and 8 mg L−1 doses, improved the functional quality. | The use of ozonated water as a sanitizing method, especially at 6 and 8 mg L−1 doses, maintained low microbial counts. | Silveira et al., 2018 [93] |
| Melon | Aqueous ozone 0.8 ppm | There are no adverse effects on SSC, color and firmness. | Reduction in total microbial counts on melon cubes (<2 log CFU g−1). | Botondi et al., 2016 [26] |
| Apple | Aqueous ozone 1.4 mg L−1 for 5 and 10 min | The ethylene production, polyphenol oxidase and peroxidase activities, and total phenol and malondialdehyde contents were reduced by aqueous ozone treatments. AO treatments delayed the quality deterioration and enhanced their antioxidant capacity. | AO treatment for 5 and 10 min achieved accepted microbial quality and, respectively, reduced total bacteria counts by 1.83 and 2.13 log10 CFUg−1 compared with the control samples. | Liu et al., 2016 [94] |
| Pineapple | Aqueous ozone 0.6, 0.9 and 1.5 ppm | The pH values of the ozone-treated samples were slightly but significantly higher than in control samples and also increased significantly over time in all samples. The quality parameters total soluble solids, ascorbic acid and total titratable acidity, color attributes and texture were not significantly different from those in the control samples. The microbial population was reduced as the ozone concentration increased. | The total plate count, total coliform and total yeast and molds were not significantly different from those in the control samples. | Nur Aida et al., 2011 [95] |
| Guava, pineapple and banana | Aqueous ozone 8 mL s−1 for 0, 10, 20 and 30 min | Total phenol and flavonoid contents of pineapple and banana increased significantly when exposed to ozone for up to 20 min, with a concomitant increase in ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) values. The opposite was observed for guava. Ozone treatment significantly decreased the ascorbic acid content of all three fruits. | Alothman et al., 2010 [96] |
| Vegetables | Treatment | Qualitative Results | Microbiological Results | References |
|---|---|---|---|---|
| Rocket | Gaseous ozone 1, 2 and 5 ppm | Fresh-cut rocket was not adversely affected by the UV-C (5, 10 and 20 kJ m−2) and ozone (1, 2 and 5 ppm) treatment, maintaining the sensory quality during cold storage. | The 20 kJ UV-C m −2 treatment was found to be better than the chlorine and gaseous ozone treatments, in terms of reducing the microbial load in fresh-cut rocket. | Gutiérrez et al., 2017 [97] |
| Green Peppers | Gaseous ozone 6.42 mg cm−3 for 15 min | The three treatments (ozone, modified atmosphere packaging and ozone + MAP) all reduced respiration rates and malondialdehyde (MDA) content compared to the control group. The enzyme activities in fresh-cut green peppers including peroxidase (POD), superoxidase dismutase (SOD) and L-phenylalanin ammonia-lyase (PAL) were induced by ozone and MAP treatments, while polyphenol oxidase (PPO) activities were inhibited. | Chen et al., 2016 [98] | |
| Lettuce, spinach and parsley | Gaseous ozone 950 μL L−1, 20 min Aqueous ozone 12 mg L−1 | AO does not affect chemical characteristics of the vegetables (chlorophyll a, chlorophyll b, ascorbic acid and total phenolic contents and antioxidant activity). GO caused significant losses in important bioactive compounds of parsley. Ascorbic acid and total phenolic contents and antioxidant activity in ozone-treated samples were 40.1, 14.4 and 41.0%, respectively, less than the control samples. | Aqueous ozone: chlorine and ozone washes resulted in average log units reductions of 2.9 and 2.0 for E. coli in the vegetables tested, respectively, while the efficiency of ozone (2.2 log) was very close to that of chlorine (2.3 log) on L. innocua. GO treatment resulted in 1.0–1.5 log reductions in the numbers of E. coli and L. innocua in parsley. | Karaca and Veliogu, 2014 [99] |
| Bell peppers | Aqueous ozone 1 μL L−1 Gaseous ozone 0.7 μL L−1 | In all the experiments, O2 continuously decreased and CO2 concentration increased. The pH value increased and a significant softening was observed in all the fruits. By day 14, L values decreased in all the fruits, with the greatest changes found in the chlorinated samples (approximately 12 units). The aqueous solutions, ozonated (1 μL L−1) and chlorine (200 μL L−1) water showed greater changes in the quality attributes with increasing washing times. The GO treatment did not affect any of the physicochemical attributes studied. | The exposure for three min to gaseous ozone reduced the mesophiles, psychrotrophes and fungal populations of the fresh-cut peppers in 2.5, 3.3 and 1.8 log units, respectively, compared to ozone treatment in water. | Horvitz et al., 2014 [100] |
| Tomato slices | Gaseous ozone 0.4 mg L−1 for 1, 3 and 5 min | The poor appearance, aroma and overall quality obtained in all treatments. | It is recommended to wash tomato slices with 0.4 mg L−1 ozonated water for 3 min only. Extending treatment duration did not improve the microbiological quality, possibly due to the extra time permitting the ozone to react with other components of the fruit tissue, undermining the antimicrobial benefits. | Aguayo et al., 2006 [101] |
| Fruits | Treatment | Qualitative Results | Microbiological Results | References |
|---|---|---|---|---|
| Papaya | Gaseous ozone 9.2 μL L−1 for 10, 20 and 30 min | Following a 20 min ozone treatment, the total phenolic content of fresh-cut papaya increased by 10.3%, while the ascorbic acid content decreased by 2.3% compared to that of untreated control fruit. | Gaseous ozone reduced microbial counts being more effective on coliforms (0.39–1.12 log10 CFUg1) than on mesophilic (0.22–0.33 log10 CFUg1) bacteria. | Yeoh et al., 2014 [102] |
| Melon | Gaseous ozone 6.34 mg m−3 | The integrity of the slices treated with gaseous ozone (GO) was preserved better than those of the others (hazelnut oil, NaClO), and no juice leakage was observed during storage. For sensorial attributes, control, NaClO and HO-treated melon slices preserved their quality for six days, whereas GO-treated samples were stored for nine days with good quality. | For microbial attributes, control, NaClO- and HO-treated melon slices were preserved their quality for six days, whereas GO-treated samples were stored for nine days with good quality. | Dilmaçünal et al., 2014 [103] |
| Cantaloupe | Gaseous ozone 5000, 20,000 and 10,000 ppm for 30 min | Gaseous ozone treatments maintained an acceptable visual quality, aroma and firmness. | Gaseous ozone 10,000 ppm for 30 min under vacuum reduced viable Salmonella. Salmonella viability loss was greater on dry exocarp surfaces than in the wetted surfaces during ozone treatment. Gaseous ozone treatment of 5000 and 20,000 ppm for 30 min reduced total coliforms, Pseudomonas fluorescens, yeast and lactic acid bacteria. | Selma et al., 2008 [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. https://doi.org/10.3390/foods10040748
Botondi R, Barone M, Grasso C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods. 2021; 10(4):748. https://doi.org/10.3390/foods10040748
Chicago/Turabian StyleBotondi, Rinaldo, Marco Barone, and Claudia Grasso. 2021. "A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables" Foods 10, no. 4: 748. https://doi.org/10.3390/foods10040748
APA StyleBotondi, R., Barone, M., & Grasso, C. (2021). A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods, 10(4), 748. https://doi.org/10.3390/foods10040748