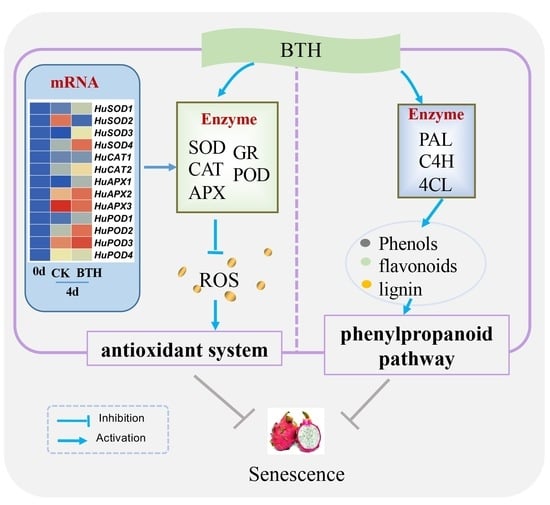
BTH Treatment Delays the Senescence of Postharvest Pitaya Fruit in Relation to Enhancing Antioxidant System and Phenylpropanoid Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Weight Loss (WL) Rate
2.3. Malondialdehyde (MDA) Content
2.4. H2O2 Content
2.5. Reduced Ascorbate (AsA) and Dehydroascorbate (DHA), Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Contents
2.6. Lignin, Total Phenolic and Flavonoids Contents
2.7. Enzymatic Activity Assays
2.8. RNA Extraction and Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
3.1. Effect of BTH Treatment on Weight Loss and MDA Content of Harvested Pitaya Fruit
3.2. H2O2 Content and Activities of CAT, APX and SOD
3.3. Activities of GR and POD, Contents of GSH and AsA and Ratios of GSH/GSSG and AsA and DHA
3.4. Activities of PAL, C4H and 4CL
3.5. Total Phenols, Flavonoids and Lignin Contents
3.6. Expression of HuSODs, HuAPXs, HuCATs and HuPODs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.L.; Meng, F.B.; Wei, X.P.; Lin, M. Postharvest dipping treatment with BABA induced resistance against rot caused by Gilbertella persicaria in red pitaya fruit. Sci. Hortic. 2019, 257, 108713. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y.H. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Tec. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Narvaez-Cuenca, C.E.; Espinal-Ruiz, M.; Restrepo-Sanchez, L.P. Heat shock reduces both chilling injury and the overproduction of reactive oxygen species in yellow pitaya (Hylocereus Megalanthus) fruits. J. Food Qual. 2011, 34, 327–332. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Mitcham, E.J. Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Sci. Agric. 2013, 70, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Lavergne, F.; Richard, C.; Saudreau, M.; Venisse, J.S.; Fumanal, B.; Goupil, P. Effect of acibenzolar-S-methyl phototransformation on its elicitation activity in tobacco cells. Plant Physiol. Biochem. 2017, 118, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Mandal, S.; Csinos, A.S.; Martinez, N.; Culbreath, A.K.; Pappu, H.R. Biological and molecular analyses of the acibenzolar-S-methyl-induced systemic acquired resistance in flue-cured tobacco against tomato spotted wilt virus. Phytopathology 2008, 98, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.H.; Deng, H.W.; Bi, Y.; Li, C.Y.; Liu, Y.Y.; Dong, B.Y. Postharvest ASM dipping and DPI pre-treatment regulated reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2015, 99, 160–167. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ge, Y.H.; Bi, Y.; Li, C.Y.; Deng, H.W.; Dong, B.Y. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014, 168, 113–119. [Google Scholar] [CrossRef]
- Cao, S.F.; Hu, Z.C.; Zheng, Y.H.; Yang, Z.F.; Lu, B.H. Effect of BTH on antioxidant enzymes, radical-scavenging activity and decay in strawberry fruit. Food Chem. 2011, 125, 145–149. [Google Scholar] [CrossRef]
- Johnson, K.B.; Smith, T.J.; Temple, T.N.; Gutierrez, E.; Elkins, R.B.; Castagnoli, S.P. Integration of acibenzolar-S-methyl with antibiotics for protection of pear and apple from fire blight caused by Erwinia amylovora. Crop Prot. 2016, 88, 149–154. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, J.; Wang, Q.; Jiang, W. Postharvest infiltration of BTH reduces infection of mango fruits (Mangifera indica L. cv. Tainong) by colletotrichum gloeosporioides and enhances resistance inducing compounds. J. Phytopathol. 2008, 156, 68–74. [Google Scholar] [CrossRef]
- Li, X.; Bi, Y.; Wang, J.J.; Dong, B.Y.; Li, H.J.; Gong, D.; Zhao, Y.; Tang, Y.M.; Yu, X.Y.; Shang, Q. BTH treatment caused physiological, biochemical and proteomic changes of muskmelon (Cucumis melo L.) fruit during ripening. J. Proteom. 2015, 120, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Lin, H.Z.; Si, Z.W.; Xia, Y.H.; Chen, W.X.; Li, X.P. Benzothiadiazole-mediated induced resistance to colletotrichum musae and delayed ripening of harvested banana fruit. J. Agric. Food Chem. 2016, 64, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Ugarte, N.; Petropoulos, I.; Friguet, B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid. Redox Signal. 2010, 13, 539–549. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hung, Y.C.; Chen, M.Y.; Lin, M.S.; Lin, H.T. Enhanced storability of blueberries by acidic electrolyzed oxidizing water application may be mediated by regulating ROS metabolism. Food Chem. 2019, 270, 229–235. [Google Scholar] [CrossRef]
- Li, T.T.; Wu, Q.X.; Zhou, Y.J.; Yun, Z.; Duan, X.W.; Jiang, Y.M. L-Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest Biol. Technol. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- You, Y.L.; Jiang, Y.M.; Sun, J.; Liu, H.; Song, L.L.; Duan, X.W. Effects of short-term anoxia treatment on browning of fresh-cut Chinese water chestnut in relation to antioxidant activity. Food Chem. 2012, 132, 1191–1196. [Google Scholar] [CrossRef]
- Zhu, S.H.; Sun, L.; Liu, M.C.; Zhou, J. Effect of nitric oxide on reactive oxygen species and antioxidant enzymes in kiwifruit during storage. J. Sci. Food Agric. 2008, 88, 2324–2331. [Google Scholar] [CrossRef]
- Jiang, G.X.; Xiao, L.; Yan, H.L.; Zhang, D.D.; Wu, F.W.; Liu, X.C.; Su, X.G.; Dong, X.H.; Wang, J.S.; Duan, X.W.; et al. Redox regulation of methionine in calmodulin affects the activity levels of senescence-related transcription factors in litchi. BBA Gen. Subj. 2017, 1861, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.Z.; Meng, X.H.; Wang, Q.; Tian, S.P. Oxidative damage of mitochondrial proteins contributes to fruit senescence: A redox proteomics analysis. J. Proteome Res. 2009, 8, 2449–2462. [Google Scholar] [CrossRef]
- Tian, S.P.; Qin, G.Z.; Li, B.Q. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.Y.; Cheng, Y.; Hou, J.B.; Zhang, J.H.; Ge, Y.H. Postharvest application of acibenzolar-S-methyl delays the senescence of pear fruit by regulating reactive oxygen species and fatty acid metabolism. J. Agric. Food Chem. 2020, 68, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.H.; Li, X.; Li, C.Y.; Tang, Q.; Duan, B.; Cheng, Y.; Hou, J.B.; Li, J.R. Effect of sodium nitroprusside on antioxidative enzymes and the phenylpropanoid pathway in blueberry fruit. Food Chem. 2019, 295, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.H.; Ma, J.H.; Xie, J.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Shivashankar, S.; Sumathi, M.; Krishnakumar, N.K.; Rao, V.K. Role of phenolic acids and enzymes of phenylpropanoid pathway in resistance of chayote fruit (Sechium edule) against infestation by melon fly, bactrocera cucurbitae. Ann. Appl. Biol. 2015, 166, 420–433. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.Y.; Gong, D.; Xue, H.L.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid s-methyl ester (BTH) promotes tuber wound healing of potato by elevation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wei, M.L.; Ge, Y.H.; Li, C.Y.; Han, X.; Qin, S.C.; Chen, Y.R.; Tang, Q.; Li, J.R. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019, 148, 228–235. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, Y.F.; Bi, Y.; Ge, Y.H.; Wang, Y.; Fan, C.F.; Li, D.Q.; Deng, H.W. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Li, S.E.; Jiang, H.; Wang, Y.; Lyu, L.; Prusky, D.; Ji, Y.; Zheng, X.L.; Bi, Y. Effect of benzothiadiazole treatment on improving the mitochondrial energy metabolism involved in induced resistance of apple fruit during postharvest storage. Food Chem. 2020, 302, 125288. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.H.; Huber, D.J.; Su, Z.H.; Hu, M.J.; Gao, Z.Y.; Li, M.; Shi, X.Q.; Zhang, Z.K. Effect of postharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chem. 2018, 243, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gong, L.; Zhang, X.Y.; Ren, A.; Gao, T.; Zhao, M.W. The regulation of methyl jasmonate on hyphal branching and GA biosynthesis in Ganoderma lucidum partly via ROS generated by NADPH oxidase. Fungal Genet. Biol. 2015, 81, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Jiang, G.; Yan, H.; Xiao, L.; Liang, H.; Zhang, D.; Jiang, Y.; Duan, X. Redox regulation of glutathione peroxidase by thioredoxin in longan fruit in relation to senescence and quality deterioration. Food Chem. 2021, 345, 128664. [Google Scholar] [CrossRef]
- Pandey, V.P.; Singh, S.; Jaiswal, N.; Awasthi, M.; Pandey, B.; Dwivedi, U.N. Papaya fruit ripening: ROS metabolism, gene cloning, characterization and molecular docking of peroxidase. J. Mol. Catal. B Enzym. 2013, 98, 98–105. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Duan, X.W.; You, Y.L.; Su, X.G.; Qu, H.X.; Joyce, D.C.; Jiang, Y.M. Influence of the nitric, oxide donor, sodium nitroprusside, on lipid peroxidation and anti-oxidant activity in pericarp tissue of longan fruit. J. Hortic. Sci. Biotechnol. 2007, 82, 467–473. [Google Scholar]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; de Oliveira, J.M.P.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Baysal, O.; Turgut, C.; Mao, G. Acibenzolar-S-methyl induced resistance to Phytophthora capsici in pepper leaves. Biol. Plant. 2005, 49, 599–604. [Google Scholar] [CrossRef]
- Faize, M.; Faize, L.; Koike, N.; Ishizaka, M.; Ishii, H. Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology 2004, 94, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, J.L.; Austin, M.B.; Stewart, C.; Noe, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Stadnik, M.J.; Buchenauer, H. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp tritici. Physiol. Mol. Plant Pathol. 2000, 57, 25–34. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. UK 2020, 10, 14685. [Google Scholar] [CrossRef]
- Li, Y.; Yu, T.; Wu, T.Q.; Wang, R.; Wang, H.M.; Du, H.; Xu, X.W.; Xie, D.S.; Xu, X.M. The dynamic transcriptome of pepper (Capsicum annuum) whole roots reveals an important role for the phenylpropanoid biosynthesis pathway in root resistance to Phytophthora capsici. Gene 2020, 728, 144288. [Google Scholar] [CrossRef]
- Li, Z.B.; Wang, N.; Wei, Y.Y.; Zou, X.R.; Jiang, S.; Xu, F.; Wang, H.F.; Shao, X.F. Terpinen-4-ol enhances disease resistance of postharvest strawberry fruit more effectively than tea tree oil by activating the phenylpropanoid metabolism pathway. J. Agric. Food Chem. 2020, 68, 6739–6747. [Google Scholar] [CrossRef]
- Shull, T.E.; Kurepa, J.; Miller, R.D.; Martinez-Ochoa, N.; Smalle, J.A. Inhibition of fusarium oxysporum f. sp. nicotianae growth by phenylpropanoid pathway intermediates. Plant Pathol. J. 2020, 36, 637–642. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.A.; Aguilera-Aguirre, S.; Vega-Arreguin, J.; Acevedo-Hernandez, G.; Tovar-Perez, E.; Stoll, A.; Herrera-Estrella, L.; Chacon-Lopez, A. Activation of the phenylpropanoid biosynthesis pathway reveals a novel action mechanism of the elicitor effect of chitosan on avocado fruit epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef]
- Deng, L.; Yin, B.; Yao, S.; Wang, W.; Zeng, K. Postharvest application of oligochitosan and chitosan reduces calyx alterations of citrus fruit induced by ethephon degreening treatment. J. Agric. Food Chem. 2016, 64, 7394–7403. [Google Scholar] [CrossRef]
- Li, C.B.; Xin, M.; Li, L.; He, X.M.; Liu, G.M.; Li, J.M.; Sheng, J.F.; Sun, J. Transcriptome profiling helps to elucidate the mechanisms of ripening and epidermal senescence in passion fruit (Passiflora edulia Sims). PLoS ONE 2020, 15, e0236535. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Ding, Y.D.; Chang, J.W.; Sun, X.H.; Zhang, L.; Wei, Q.J.; Cheng, Y.J.; Chen, L.L.; Xu, J.; Deng, X.X. Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches. J. Exp. Bot. 2014, 65, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, C.A.; Azocar, C.; Ramos, P.; Perez-Diaz, R.; Sepulveda, G.; Moya-Leon, M.A. Photooxidative stress activates a complex multigenic response integrating the phenylpropanoid pathway and ethylene, leading to lignin accumulation in apple (Malus domestica Borkh.) fruit. Hortic. Res. Engl. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Zhu, X.; Zheng, W.; Li, F.; Xiao, S.; Duan, X. BTH Treatment Delays the Senescence of Postharvest Pitaya Fruit in Relation to Enhancing Antioxidant System and Phenylpropanoid Pathway. Foods 2021, 10, 846. https://doi.org/10.3390/foods10040846
Ding X, Zhu X, Zheng W, Li F, Xiao S, Duan X. BTH Treatment Delays the Senescence of Postharvest Pitaya Fruit in Relation to Enhancing Antioxidant System and Phenylpropanoid Pathway. Foods. 2021; 10(4):846. https://doi.org/10.3390/foods10040846
Chicago/Turabian StyleDing, Xiaochun, Xiaoyang Zhu, Wang Zheng, Fengjun Li, Shuangling Xiao, and Xuewu Duan. 2021. "BTH Treatment Delays the Senescence of Postharvest Pitaya Fruit in Relation to Enhancing Antioxidant System and Phenylpropanoid Pathway" Foods 10, no. 4: 846. https://doi.org/10.3390/foods10040846
APA StyleDing, X., Zhu, X., Zheng, W., Li, F., Xiao, S., & Duan, X. (2021). BTH Treatment Delays the Senescence of Postharvest Pitaya Fruit in Relation to Enhancing Antioxidant System and Phenylpropanoid Pathway. Foods, 10(4), 846. https://doi.org/10.3390/foods10040846