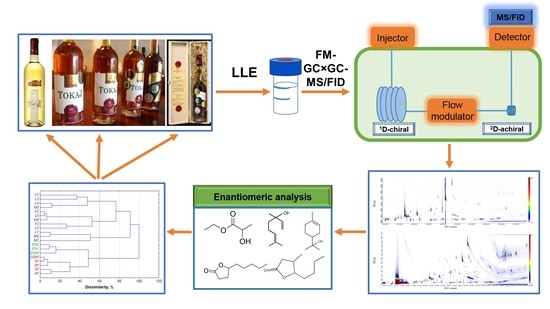
Classification of Botrytized Wines Based on Producing Technology Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Sample Preparation
2.4. Instrumentation
3. Results
3.1. Ethyl Lactate
3.2. Terpenes
3.3. Lactones
3.4. Hierarchical Clustering Analysis of the Wine Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GC×GC | comprehensive two-dimensional gas chromatography |
| RT | retention time |
| RI | retention index |
References
- Magyar, I.; Soós, J. Botrytized wines–current perspectives. Int. J. Wine Res. 2016, 8, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.S.; Cilindre, C.; Liger-Belair, G.; Jeandet, P.; Hertkorn, N.; Schmitt-Kopplin, P. Metabolic Influence of Botrytis cinerea Infection in Champagne Base Wine. J. Agric. Food Chem. 2011, 59, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, J.; Riberau-Gayon, P.; Seguin, G. Botrytis cinerea in enology. In Biology of Botrytis; Coley-Smith, K.J.R., Verhoeff, W.R., Jarvis, Eds.; Academic Press: London, UK, 1980; pp. 251–274. [Google Scholar]
- Sipiczki, M. Yeasts in Botrytized Wine Making. Yeasts Prod. Wine 2019. [Google Scholar] [CrossRef]
- Csoma, H.; Kállai, Z.; Antunovics, Z.; Czentye, K.; Sipiczki, M. Vinification without Saccharomyces: Interacting Osmotolerant and “Spoilage” Yeast Communities in Fermenting and Ageing Botrytised High-Sugar Wines (Tokaj Essence). Microorganisms 2021, 9, 19. [Google Scholar] [CrossRef]
- Eftimova, Z.; Eftimová, J.; Balážová, Ľ. Antioxidant activity of tokaj essence. Potravinarstvo 2018, 12, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Furdikova, K.; Machynakova, A.; Drtilova, T.; Spanik, I. Comparison of Different Categories of Slovak Tokaj Wines in Terms of Profiles of Volatile Organic Compounds. Molecules 2020, 25, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosi, E.; Fedrizzi, B.; Azzolini, M.; Finato, F.; Simonato, B.; Zapparoli, G. Effects of noble rot on must composition and aroma profile of Amarone wine produced by the traditional grape withering protocol. Food Chem. 2012, 130, 370–375. [Google Scholar] [CrossRef]
- Thibon, C.; Shinkaruk, S.; Jourdes, M.; Bennetau, B.; Dubourdieu, D.; Tominaga, T. Aromatic potential of botrytized white wine grapes: Identification and quantification of new cysteine-S-conjugate flavor precursors. Anal. Chim. Acta 2010, 660, 190–196. [Google Scholar] [CrossRef]
- Tominaga, T.; Niclass, Y.; Frerot, E.; Dubourdieu, D. Stereoisomeric distribution of 3-mercaptohexan-1-ol and 3-mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (var. Sauvignon blanc and Semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef]
- Ribeiro, C.; Gonçalves, R.; Tiritan, M. Separation of Enantiomers Using Gas Chromatography: Application in Forensic Toxicology, Food and Environmental Analysis. Crit. Rev. Anal. Chem. 2020, 1–25. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R. Chirality and the Nature of Food Authenticity of Aroma. Acta Sci. Polon. Technol. 2006, 5, 21–36. [Google Scholar]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Cifuentes, A. Chiral analysis in food science. Trac-Trend Anal. Chem. 2020, 123, 151–171. [Google Scholar] [CrossRef]
- Mu, B.; Zhu, Y.; Lv, H.P.; Yan, H.; Peng, Q.H.; Lin, Z. The enantiomeric distributions of volatile constituents in different tea cultivars. Food Chem. 2018, 265, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, B.; Orlandini, S.; Goodarzi, M.; Caprini, C.; Gotti, R.; Furlanetto, S. Chiral cyclodextrin-modified micellar electrokinetic chromatography and chemometric techniques for green tea samples origin discrimination. Talanta 2016, 150, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Taniguchi, M.; Fukusaki, E. High-sensitive liquid chromatography-tandem mass spectrometry-based chiral metabolic profiling focusing on amino acids and related metabolites. J. Biosci. Bioeng. 2019, 127, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, A.; Siano, G.; Quintas, P.; Filgueira, M.R.; Castells, C.B. Chiral x achiral multidimensional liquid chromatography. Application to the enantioseparation of dintitrophenyl amino acids in honey samples and their fingerprint classification. J. Chromatogr. A 2020, 1614, 460729. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Pollini, L.; Cossignani, L. Varietal Authentication of Extra Virgin Olive Oils by Triacylglycerols and Volatiles Analysis. Foods 2019, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Cordero, C.; Schmarr, H.G.; Reichenbach, S.E.; Bicchi, C. Current Developments in Analyzing Food Volatiles by Multidimensional Gas Chromatographic Techniques. J. Agric. Food Chem. 2018, 66, 2226–2236. [Google Scholar] [CrossRef]
- Krupcik, J.; Gorovenko, R.; Spanik, I.; Armstrong, D.W.; Sandra, P. Enantioselective comprehensive two-dimensional gas chromatography of lavender essential oil. J. Sep. Sci. 2016, 39, 4765–4772. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P.; Cornwell, C. Application of comprehensive two-dimensional gas chromatography (GC× GC) to the enantioselective analysis of essential oils. J. Sep. Sci. 2001, 24, 823–830. [Google Scholar] [CrossRef]
- Wang, M.; Marriott, P.J.; Chan, W.H.; Lee, A.W.M.; Huie, C.W. Enantiomeric separation and quantification of ephedrine-type alkaloids in herbal materials by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2006, 1112, 361–368. [Google Scholar] [CrossRef]
- Machynakova, A.; Khvalbota, L.; Furdikova, K.; Drtilova, T.; Spanik, I. Characterization of volatile organic compounds in Slovak Tokaj wines. J. Food Nutr. Res. Slov. 2019, 58, 307–318. [Google Scholar]
- Vyviurska, O.; Spanik, I. Assessment of Tokaj varietal wines with comprehensive two-dimensional gas chromatography coupled to high resolution mass spectrometry. Microchem. J. 2020, 152, 104385. [Google Scholar] [CrossRef]
- Pazitna, A.; Dzurova, J.; Spanik, I. Enantiomer Distribution of Major Chiral Volatile Organic Compounds in Selected Types of Herbal Honeys. Chirality 2014, 26, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kaunzinger, A.; Wüst, M.; Gröbmiller, H.; Burow, S.; Hemmrich, U.; Dietrich, A.; Beck, T.; Hener, U.; Mosandl, A.; Rapp, A. Enantiomer distribution of ethyl lactate—A new criterion for quality assurance of wine. Z. Für Lebensm. Unters. Forsch. 1996, 203, 499–500. [Google Scholar] [CrossRef]
- Lloret, A.; Boido, E.; Lorenzo, D.; Medina, K.; Carrau, F.; Dellacassa, E.; Versini, G. Aroma variation in tannat wines: Effect of malolactic fermentation on ethyl lactate level and its enantiomeric distribution. Ital. J. Food Sci. 2002, 14, 175–180. [Google Scholar]
- Pozo-Bayón, M.; G-Alegría, E.; Polo, M.; Tenorio, C.; Martín-Álvarez, P.; Calvo De La Banda, M.; Ruiz-Larrea, F.; Moreno-Arribas, M. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Joseph, C.M.L.; Allen, G.; Benson, A.K.; Mills, D.A. Next-Generation Sequencing Reveals Significant Bacterial Diversity of Botrytized Wine. PLoS ONE 2012, 7, e36357. [Google Scholar] [CrossRef] [PubMed]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of different techniques of malolactic fermentation induction on diacetyl metabolism and biosynthesis of selected aromatic esters in cool-climate grape wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.; Relva, A.M.; da Silva, M.D.R.G.; Freitas, A.M.C. Different multidimensional chromatographic approaches applied to the study of wine malolactic fermentation. J. Chromatogr. A 2003, 995, 161–169. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety ‘Irsai Oliver’(Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Papadopoulou, D. Inhibition of the decline of volatile esters and terpenols during oxidative storage of Muscat-white and Xinomavro-red wine by caffeic acid and N-acetyl-cysteine. Food Chem. 2005, 93, 485–492. [Google Scholar] [CrossRef]
- Bock, G.; Benda, I.; Schreier, P. Biotransformation of Linalool by Botrytis-Cinerea. J. Food Sci. 1986, 51, 659–662. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010, 120, 205–216. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. J. Food Compos. Anal. 2008, 21, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Tao, Y.S.; Wu, Y.; An, R.Y.; Yue, Z.Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: London, UK, 2008. [Google Scholar]
- Cerdan, T.G.; Goni, D.T.; Azpilicueta, C.A. Accumulation of volatile compounds during ageing of two red wines with different composition. J. Food Eng. 2004, 65, 349–356. [Google Scholar] [CrossRef]
- Cooke, R.C.; Van Leeuwen, K.A.; Capone, D.L.; Gawel, R.; Elsey, G.M.; Sefton, M.A. Odor detection thresholds and enantiomeric distributions of several 4-alkyl substituted γ-lactones in Australian red wine. J. Agric. Food Chem. 2009, 57, 2462–2467. [Google Scholar] [CrossRef]
- Staniatopoulos, P.; Brohan, E.; Prevost, C.; Siebert, T.E.; Herderich, M.; Darriet, P. Influence of Chirality of Lactones on the Perception of Some Typical Fruity Notes through Perceptual Interaction Phenomena in Bordeaux Dessert Wines. J. Agric. Food Chem. 2016, 64, 8160–8167. [Google Scholar] [CrossRef]
- Suomalainen, H.; Nykänen, L. Composition of whisky flavour. Process Biochem. 1970, 5, 13–18. [Google Scholar]
- Shimotori, Y.; Hoshi, M.; Okabe, H.; Miyakoshi, T.; Kanamoto, T.; Nakashima, H. Synthesis, odour characteristics and antibacterial activities of the stereoisomeric forms of whisky lactone and its thiono analogues. Flavour Frag. J. 2017, 32, 29–35. [Google Scholar] [CrossRef]
- Arapitsas, P.; Antonopoulos, A.; Stefanou, E.; Dourtoglou, V. Artificial aging of wines using oak chips. Food Chem. 2004, 86, 563–570. [Google Scholar] [CrossRef]
- Spillman, P.J.; Sefton, M.A.; Gawel, R. The effect of oak wood source, location of seasoning and coopering on the composition of volatile compounds in oak-matured wines. Aust. J. Grape Wine Res. 2004, 10, 216–226. [Google Scholar] [CrossRef]
- Moreno, N.J.; Azpilicueta, C.A. Binding of oak volatile compounds by wine lees during simulation of wine ageing. LWT-Food Sci. Technol. 2007, 40, 619–624. [Google Scholar] [CrossRef]
- Brown, R.C.; Sefton, M.A.; Taylor, D.K.; Elsey, G.M. An odour detection threshold determination of all four possible stereoisomers of oak lactone in a white and a red wine. Aust. J. Grape Wine Res. 2006, 12, 115–118. [Google Scholar] [CrossRef]
- del Alamo-Sanza, M.; Nevares, I.; Martinez-Gil, A.; Rubio-Breton, P.; Garde-Cerdan, T. Impact of long bottle aging (10 years) on volatile composition of red wines micro-oxygenated with oak alternatives. LWT-Food Sci. Technol. 2019, 101, 395–403. [Google Scholar] [CrossRef]
- Perez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Determination of volatile compounds in hydroalcoholic extracts of French and American oak weed. Am. J. Enol. Viticult. 1999, 50, 162–165. [Google Scholar]
- Liu, N.; Koot, A.; Hettinga, K.; de Jong, J.; van Ruth, S.M. Portraying and tracing the impact of different production systems on the volatile organic compound composition of milk by PTR-(Quad)MS and PTR-(ToF)MS. Food Chem. 2018, 239, 201–207. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]



| Code | Year | Producer | Code | Year | Producer |
|---|---|---|---|---|---|
| ES1 | 1999 | TOKAJ & CO. | 5P7 | 2000 | Zlatý Strapec |
| ES2 | 2000 | J & J OSTROŽOVIČ | 5P8 | 2003 | J & J OSTROŽOVIČ |
| GP30 | 2016 | J & J OSTROŽOVIČ | 5P9 | 2003 | J & J OSTROŽOVIČ |
| GP90 | 2016 | J & J OSTROŽOVIČ | 5P10 | 2004 | J & J OSTROŽOVIČ |
| 2P1 | 1989 | TOKAJ & CO. | 6P1 | 1977 | Zlatý Strapec |
| 2P2 | 1990 | TOKAJ & CO. | 6P2 | 1983 | Zlatý Strapec |
| 3P1 | 1988 | Zlatý Strapec | 6P3 | 1989 | J & J OSTROŽOVIČ |
| 3P2 | 1990 | TOKAJ & CO. | 6P4 | 1989 | TOKAJ & CO. |
| 3P3 | 1995 | J & J OSTROŽOVIČ | 6P5 | 1999 | J & J OSTROŽOVIČ |
| 3P4 | 1995 | Zlatý Strapec | 6P6 | 2002 | J & J OSTROŽOVIČ |
| 3P5 | 1999 | J & J OSTROŽOVIČ | 6P7 | 2003 | J & J OSTROŽOVIČ |
| 3P6 | 2000 | Zlatý Strapec | 6P8 | 2006 | TOKAJ & CO. |
| 3P7 | 2009 | TOKAJ & CO. | F1 | 2014 | J & J OSTROŽOVIČ |
| 4P1 | 1993 | Zlatý Strapec | F2 | 2015 | TOKAJ & CO. |
| 4P2 | 1995 | TOKAJ & CO. | F3 | 2015 | J & J OSTROŽOVIČ |
| 4P3 | 2000 | Zlatý Strapec | M1 | 2016 | J & J OSTROŽOVIČ |
| 4P4 | 2002 | J & J OSTROŽOVIČ | M2 | 2016 | J & J OSTROŽOVIČ |
| 4P5 | 2004 | J & J OSTROŽOVIČ | M4 | 2015 | J & J OSTROŽOVIČ |
| 4P6 | 2009 | TOKAJ & CO. | M5 | 2015 | TOKAJ & CO. |
| 5P1 | 1959 | Zlatý Strapec | L1 | 2015 | J & J OSTROŽOVIČ |
| 5P2 | 1972 | Zlatý Strapec | L2 | 2015 | TOKAJ & CO. |
| 5P3 | 1989 | J & J OSTROŽOVIČ | L3 | 2015 | TOKAJ & CO. |
| 5P4 | 1990 | TOKAJ & CO. | L4 | 2015 | J & J OSTROŽOVIČ |
| 5P5 | 1993 | J & J OSTROŽOVIČ | L5 | 2015 | J & J OSTROŽOVIČ |
| 5P6 | 1993 | Zlatý Strapec | - | - | - |
| Compounds | RT1, min | RT2, s | RI | Resolution |
|---|---|---|---|---|
| R-(+)-ethyl lactate | 44.288 | 1.491 | 972 | - |
| S-(-)-ethyl lactate | 44.988 | 1.491 | 979 | 3.29 |
| R-(-)-linalool | 60.388 | 1.177 | 1204 | - |
| S-(+)-linalool | 60.688 | 1.177 | 1210 | 1.56 |
| R-(+)-α-terpineol | 68.288 | 1.491 | 1322 | - |
| S-(-)-α-terpineol | 68.588 | 1.491 | 1328 | 2.59 |
| cis-whiskey lactone | 78.788 | 1.648 | 1506 | - |
| trans-whiskey lactone | 81.488 | 1.805 | 1543 | 11.00 |
| R-γ-nonalactone | 84.388 | 1.833 | 1592 | - |
| S-γ-nonalactone | 84.588 | 1.833 | 1595 | 8.20 |
| Code | Enantiomer Ratio [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R-(+)-Ethyl Lactate | S-(-)-Ethyl Lactate | R-(-)-Linalool | S-(+)-Linalool | R-(+)-α-Terpineol | S-(-)-α-Terpineol | R-γ-Nonalactone | S-γ-Nonalactone | trans-Whiskey Lactone | cis-Whiskey Lactone | |
| Essences | ||||||||||
| ES1 | 24 | 76 | - | - | - | - | 61 | 39 | 38 | 62 |
| ES2 | 30 | 70 | - | - | - | - | 74 | 26 | 38 | 62 |
| Wines fermented with grape skins | ||||||||||
| GP30 | 11 | 89 | - | - | - | - | 77 | 23 | 24 | 76 |
| GP90 | 28 | 72 | - | - | - | - | 71 | 29 | 34 | 66 |
| Botrytized wines | ||||||||||
| 2P1 | 21 | 79 | - | - | - | - | 70 | 30 | 43 | 57 |
| 2P2 | 14 | 86 | - | - | - | - | 70 | 30 | - | - |
| 3P1 | 27 | 73 | - | - | - | - | 69 | 31 | 46 | 54 |
| 3P2 | 24 | 76 | - | - | - | - | 66 | 34 | 48 | 52 |
| 3P3 | 15 | 85 | - | - | - | - | 71 | 29 | 39 | 61 |
| 3P4 | 22 | 78 | - | - | 45 | 55 | 61 | 39 | 36 | 64 |
| 3P5 | 14 | 86 | - | - | - | - | 77 | 23 | 36 | 64 |
| 3P6 | 18 | 82 | - | - | - | - | 75 | 25 | 44 | 56 |
| 3P7 | 70 | 30 | 43 | 57 | - | - | - | - | 48 | 52 |
| 4P1 | 35 | 65 | - | - | - | - | - | - | 40 | 60 |
| 4P2 | 24 | 76 | - | - | - | - | - | - | 39 | 61 |
| 4P3 | 20 | 80 | - | - | - | - | 61 | 39 | 43 | 57 |
| 4P4 | 32 | 68 | - | - | - | - | - | - | 39 | 61 |
| 4P5 | 20 | 80 | - | - | - | - | 68 | 32 | 42 | 58 |
| 4P6 | 36 | 64 | 39 | 61 | 51 | 49 | - | - | 46 | 54 |
| 5P1 | 38 | 62 | - | - | - | - | - | - | 43 | 57 |
| 5P2 | 24 | 76 | - | - | - | - | - | - | 27 | 73 |
| 5P3 | 26 | 74 | - | - | - | - | - | - | 46 | 54 |
| 5P4 | 21 | 79 | - | - | - | - | 65 | 35 | 37 | 63 |
| 5P5 | 20 | 80 | - | - | - | - | 58 | 42 | 46 | 54 |
| 5P6 | 25 | 75 | - | - | - | - | 64 | 36 | 42 | 58 |
| 5P7 | 22 | 78 | - | - | - | - | 70 | 30 | 40 | 60 |
| 5P8 | 43 | 57 | - | - | 52 | 48 | - | - | 33 | 67 |
| 5P9 | 42 | 58 | - | - | 51 | 49 | - | - | 32 | 68 |
| 5P10 | 41 | 59 | - | - | - | - | 74 | 26 | 33 | 67 |
| 6P1 | 29 | 71 | - | - | 48 | 52 | 71 | 29 | 39 | 61 |
| 6P2 | 25 | 75 | - | - | - | - | - | - | 40 | 60 |
| 6P3 | 54 | 46 | - | - | - | - | - | - | 58 | 42 |
| 6P4 | 15 | 85 | - | - | - | - | - | - | 37 | 63 |
| 6P5 | 23 | 77 | - | - | - | - | 75 | 25 | 38 | 62 |
| 6P6 | 27 | 73 | - | - | 36 | 64 | 71 | 29 | 38 | 62 |
| 6P7 | 30 | 70 | - | - | 44 | 56 | 80 | 20 | 39 | 61 |
| 6P8 | 81 | 19 | - | - | 54 | 46 | 58 | 42 | 42 | 58 |
| Varietal wines | ||||||||||
| F1 | 77 | 23 | - | - | - | - | 73 | 27 | - | - |
| F2 | 79 | 21 | - | - | 55 | 45 | - | - | 42 | 58 |
| F3 | 91 | 9 | 52 | 48 | 58 | 42 | 72 | 28 | - | - |
| M1 | - | - | 56 | 44 | 67 | 33 | - | - | - | - |
| M2 | - | - | 48 | 52 | 63 | 37 | 63 | 37 | - | - |
| M4 | 83 | 17 | 51 | 49 | 59 | 41 | 68 | 32 | - | - |
| M5 | 73 | 27 | 40 | 60 | 54 | 46 | - | - | - | - |
| L1 | - | - | 38 | 62 | 59 | 41 | 74 | 26 | - | - |
| L2 | 88 | 12 | 37 | 63 | - | - | 64 | 36 | - | - |
| L3 | 50 | 50 | 47 | 53 | 54 | 46 | - | - | 53 | 47 |
| L4 | 91 | 9 | 41 | 59 | 56 | 44 | - | - | - | - |
| L5 | 90 | 10 | 45 | 55 | 56 | 44 | 71 | 29 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyviurska, O.; Koljančić, N.; Thai, H.A.; Gorovenko, R.; Špánik, I. Classification of Botrytized Wines Based on Producing Technology Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography. Foods 2021, 10, 876. https://doi.org/10.3390/foods10040876
Vyviurska O, Koljančić N, Thai HA, Gorovenko R, Špánik I. Classification of Botrytized Wines Based on Producing Technology Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography. Foods. 2021; 10(4):876. https://doi.org/10.3390/foods10040876
Chicago/Turabian StyleVyviurska, Olga, Nemanja Koljančić, Ha Anh Thai, Roman Gorovenko, and Ivan Špánik. 2021. "Classification of Botrytized Wines Based on Producing Technology Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography" Foods 10, no. 4: 876. https://doi.org/10.3390/foods10040876
APA StyleVyviurska, O., Koljančić, N., Thai, H. A., Gorovenko, R., & Špánik, I. (2021). Classification of Botrytized Wines Based on Producing Technology Using Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography. Foods, 10(4), 876. https://doi.org/10.3390/foods10040876