Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Samples
2.2. Chemical Analyses
2.3. Spectra Collection
2.4. Data Analysis
3. Results and Discussion
3.1. Chemical Parameters
3.2. Spectral Features
3.3. Principal Component Analysis
3.4. Regression Models
3.5. Regression Model Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casson, A.; Beghi, R.; Giovenzana, V.; Fiorindo, I.; Tugnolo, A.; Guidetti, R. Visible near infrared spectroscopy as a green technology: An environmental impact comparative study on olive oil analyses. Sustainability 2019, 11, 2611. [Google Scholar] [CrossRef] [Green Version]
- Arvanitoyannis, I.S.; Kassaveti, A. Current and potential uses of composted olive oil waste. Int. J. Food Sci. Technol. 2017, 42, 281–295. [Google Scholar] [CrossRef]
- Banias, G.; Achillas, C.; Vlachokostas, C. Environmental impacts in the life cycle of olive oil: A literature review. J. Sci. Food Agric. 2017, 97, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste. Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: Oxford, UK, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Pantziaros, A.G.; Trachili, X.A.; Zentelis, A.D.; Sygouni, V.; Paraskeva, C.A. A new olive oil production scheme with almost zero wastes. Biomass Conv. Bioref. 2020. [Google Scholar] [CrossRef]
- Rosello-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non- conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Palagos, B.; Peña-Rodríguez, F.; Roger, J.M. Calibration transfer of intact olive NIR spectra between a pre-dispersive instrument and a portable spectrometer. Comp. Electron. Agric. 2013, 96, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Sánchez, N.; Gómez-Del-Campo, M. From NIR spectra to singular wavelengths for the estimation of the oil and water contents in olive fruits. Grasas Aceites 2018, 69, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Morrone, L.; Neri, L.; Cantini, C.; Alfei, B.; Rotondi, A. Study of the combined effects of ripeness and production area on Bosana oil’s quality. Food Chem. 2018, 245, 1098–1104. [Google Scholar] [CrossRef]
- Correa, E.C.; Roger, J.M.; Lleó, L.; Hernández-Sánchez, N.; Barreiro, P.; Diezma, B. Optimal management of oil content variability in olive mill batches by NIR spectroscopy. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Giovenzana, V.; Beghi, R.; Romaniello, R.; Tamborrino, A.; Guidetti, R.; Leone, A. Use of visible and near infrared spectroscopy with a view to on-line evaluation of oil content during olive processing. Biosyst. Eng. 2018, 172, 102–109. [Google Scholar] [CrossRef]
- Bianchi, B.; Tamborrino, A.; Giametta, F.; Squeo, G.; Difonzo, G.; Catalano, P. Modified rotating reel for malaxer machines: Assessment of rheological characteristics, energy consumption, temperature profile, and virgin olive oil quality. Foods 2020, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Squeo, G.; Brunetti, L.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Catalano, P.; Bianchi, B. Influence of the feed pipe position of an industrial scale two-phase decanter on extraction efficiency and chemical-sensory characteristics of virgin olive oil. J. Sci. Food Agric. 2018, 98, 4279–4286. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Qu, J.H.; Liu, D.; Cheng, J.H.; Sun, D.W.; Ma, J.; Pu, H.; Zeng, X.A. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef]
- Leon, L.; Garrido, A.; Downey, G. Parent and harvest year effects on near-infrared reflectance spectroscopic analysis of olive (Olea europaea L.) fruit traits. J. Agric. Food Chem. 2004, 52, 4957–4962. [Google Scholar] [CrossRef] [PubMed]
- Saha, U.; Jackson, D. Analysis of moisture, oil, and fatty acid composition of olives by near-infrared spectroscopy: Development and validation calibration models. J. Sci. Food Agric. 2018, 98, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.J.; Camino, M.D.P. Prediction of quality of intact olives by near infrared spectroscopy. Eur. J. Lipid Sci. Technol. 2010, 112, 1209–1217. [Google Scholar] [CrossRef]
- Bellincontro, A.; Taticchi, A.; Servili, M.; Esposto, S.; Farinelli, D.; Mencarelli, F. Feasible application of a portable NIR-AOTF tool for on-field prediction of phenolic compounds during the ripening of olives for oil production. J. Agric. Food Chem. 2012, 60, 2665–2673. [Google Scholar] [CrossRef]
- Cirilli, M.; Bellincontro, A.; Urbani, S.; Servili, M.; Esposto, S.; Mencarelli, F.; Muleo, R. On-field monitoring of fruit ripening evolution and quality parameters in olive mutants using a portable NIR-AOTF device. Food Chem. 2016, 199, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Azizian, H.; Mossoba, M.M.; Fardin-Kia, A.R.; Karunathilaka, S.R.; Kramer, J.K.G. Developing FT-NIR and PLS1 methodology for predicting adulteration in representative varieties/blends of extra virgin olive oils. Lipids 2016, 51, 1309–1321. [Google Scholar] [CrossRef]
- Comino, F.; Ayora-Cañada, M.J.; Aranda, V.; Díaz, A.; Domínguez-Vidal, A. Near-infrared spectroscopy and X-ray fluorescence data fusion for olive leaf analysis and crop nutritional status determination. Talanta 2018, 188, 676–684. [Google Scholar] [CrossRef]
- Tugnolo, A.; Giovenzana, V.; Beghi, R.; Grassi, S.; Alamprese, C.; Casson, A.; Casiraghi, E.; Guidetti, R. A diagnostic visible/near infrared tool for a fully automated olive ripeness evaluation in a view of a simplified optical system. Comput. Electron. Agr. 2020, 105887. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed.; Official Method 934.06. Moisture in Dried Fruits; Journal of AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): Collaborative study. J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorini, M.; Cherubini, C.; Mugelli, M.; Gianni, G.; Trapani, S.; Zanoni, B. Relationship between the oil and sugar content in olive oil fruits from Moraiolo and Leccino cultivars during ripening. Sci. Hort. 2011, 129, 919–921. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- Rabatel, G.; Marini, F.; Walczak, B.; Roger, J.M. VSN: Variable sorting for normalization. J. Chemom. 2020, 34, 1–16. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Roggo, Y.; Duponchel, L.; Ruckebus ch, C.; Huvenne, J.P. Statistical tests for comparison of quantitative and qualitative models developed with near infrared spectral data. J. Mol. Struct. 2003, 654, 253–262. [Google Scholar] [CrossRef]
- Fearn, T. Comparing standard deviations. NIR News 1996, 7, 5–6. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medicinal Products. Note for Guidance on the Use of Near Infrared Spectroscopy by the Pharmaceutical Industry and the Data Requirements for New Submissions and Variations. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-use-near-infrared-spectroscopy-pharmaceutical-industry-data-requirements-new_en.pdf (accessed on 15 April 2021).
- Trapani, S.; Migliorini, M.; Cecchi, L.; Giovenzana, V.; Beghi, R.; Canuti, V.; Fia, G.; Zanoni, B. Feasibility of filter-based NIR spectroscopy for the routine measurement of olive oil fruit ripening indices. Eur. J. Lipid Sci. Technol. 2017, 119, 1600239. [Google Scholar] [CrossRef]
- de la Casa, J.A.; Castro, E. Fuel savings and carbon dioxide emission reduction in a fired clay bricks production plant using olive oil wastes: A simulation study. J. Clean Prod. 2018, 185, 230–238. [Google Scholar] [CrossRef]
- Fernández-Espinosa, A.J. Combining PLS regression with portable NIR spectroscopy to on-line monitor quality parameters in intact olives for determining optimal harvesting time. Talanta 2016, 148, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Giovenzana, V.; Beghi, R.; Civelli, R.; Trapani, S.; Migliorini, M.; Cini, E.; Zanoni, A.; Guidetti, R. Rapid determination of crucial parameters for the optimization of milling process by using visible/near infrared spectroscopy on intact olives and olive paste. Ital. J. Food Sci. 2017, 29, 357–369. [Google Scholar] [CrossRef]
- Dupuy, N.; Galtier, O.; Le Dréau, Y.; Pinatel, C.; Kister, J.; Artaud, J. Chemometric analysis of combined NIR and MIR spectra to characterize French olives. Eur. J. Lipid Sci. Technol. 2010, 112, 463–475. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Peña-Rodríguez, F. On-line versus off-line NIRS analysis of intact olives. LWT Food Sci. Technol. 2014, 56, 363–369. [Google Scholar] [CrossRef]
- Beghi, R.; Giovenzana, V.; Marai, S.; Guidetti, R. Rapid monitoring of grape withering using visible near-infrared spectroscopy. J. Sci. Food Agric. 2015, 95, 3144–3149. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Zhao, C.; Zhang, B. A comparative study for the quantitative determination of soluble solids content, pH and firmness of pears by Vis/NIR spectroscopy. J. Food Eng. 2013, 116, 324–332. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, W.; Fan, S.; Li, J.; Chen, L. Effect of spectral measurement orientation on online prediction of soluble solids content of apple using Vis/NIR diffuse reflectance. Infrared Phys. Technol. 2019, 97, 467–477. [Google Scholar] [CrossRef]
- Sánchez, M.T.; De La Haba, M.J.; Benítez-López, M.; Fernández-Novales, J.; Garrido-Varo, A.; Pérez-Marín, D. Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J. Food Eng. 2012, 110, 102–108. [Google Scholar] [CrossRef]
- Barnaba, F.E.; Bellincontro, A.; Mencarelli, F. Portable NIR-AOTF spectroscopy combined with winery FTIR spectroscopy for an easy, rapid, in-field monitoring of Sangiovese grape quality. J. Sci. Food Agric. 2014, 94, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Carbas, B.; Machado, N.; Oppolzer, D.; Queiroz, M.; Brites, C.; Rosa, E.A.S.; Barros, A.I.R.N.A. Prediction of phytochemical composition, in vitro antioxidant activity and individual phenolic compounds of common beans using MIR and NIR spectroscopy. Food Bioproc. Technol. 2020, 13, 962–977. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S. Rapid determination of antioxidant compounds and antioxidant activity of Sudanese karkade (Hibiscus sabdariffa L.) using near infrared spectroscopy. Food Anal. Methods 2016, 9, 1228–1236. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI Way. In Near Infrared Spectroscopy: The Future Waves; Davies, P.C., Williams, A.M.C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]



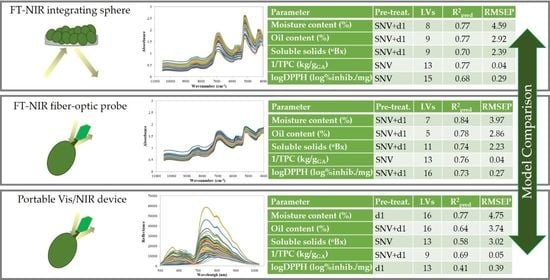
| Calibration | Cross-Validation | Prediction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | NIR System | Pre-treatment | LVs | R2cal | RMSEC | R2cv | RMSECV | R2pred | RMSEP |
| Moisture content (%) | Sphere | SNV+d1 | 8 | 0.92 | 2.67 | 0.85 | 3.66 | 0.77 | 4.59 |
| Probe | SNV+d1 | 7 | 0.88 | 3.56 | 0.85 | 3.87 | 0.84 | 3.97 | |
| Portable | d1 | 16 | 0.87 | 3.68 | 0.77 | 4.77 | 0.77 | 4.75 | |
| Oil content (%) | Sphere | SNV+d1 | 9 | 0.93 | 1.62 | 0.82 | 2.62 | 0.77 | 2.92 |
| Probe | SNV+d1 | 5 | 0.79 | 2.87 | 0.77 | 2.99 | 0.78 | 2.86 | |
| Portable | SNV+d1 | 16 | 0.81 | 2.72 | 0.67 | 3.58 | 0.64 | 3.74 | |
| Soluble solids (°Bx) | Sphere | SNV+d1 | 9 | 0.90 | 1.45 | 0.75 | 2.36 | 0.70 | 2.39 |
| Probe | SNV+d1 | 11 | 0.87 | 1.66 | 0.80 | 2.06 | 0.74 | 2.23 | |
| Portable | SNV | 13 | 0.79 | 2.11 | 0.75 | 2.34 | 0.58 | 3.02 | |
| 1/TPC (kg/gGA) | Sphere | SNV | 13 | 0.89 | 0.04 | 0.81 | 0.04 | 0.77 | 0.04 |
| Probe | SNV+d1 | 13 | 0.87 | 0.04 | 0.76 | 0.05 | 0.76 | 0.04 | |
| Portable | SNV+d1 | 9 | 0.83 | 0.05 | 0.79 | 0.05 | 0.69 | 0.05 | |
| logDPPH• (log % inhib./mg) | Sphere | SNV | 15 | 0.84 | 0.20 | 0.68 | 0.29 | 0.68 | 0.29 |
| Probe | SNV+d1 | 16 | 0.93 | 0.14 | 0.79 | 0.24 | 0.73 | 0.27 | |
| Portable | d1 | 13 | 0.79 | 0.23 | 0.72 | 0.27 | 0.41 | 0.39 | |
| Parameter | SELref | NIR System | SELNIR | SEP | NIR System | ||
|---|---|---|---|---|---|---|---|
| Sphere | Probe | Portable Device | |||||
| Moisture content (%) | 2.00 | Sphere | 4.41 | 4.56 | - | * | n.s. |
| Probe | 3.21 | 3.99 | * | - | * | ||
| Portable | 4.49 | 4.72 | n.s. | * | - | ||
| Oil content (%) | 2.29 | Sphere | 3.13 | 2.94 | - | n.s. | * |
| Probe | 2.18 | 2.88 | n.s. | - | * | ||
| Portable | 2.95 | 3.77 | * | * | - | ||
| Soluble solids (°Bx) | 1.02 | Sphere | 2.21 | 2.41 | - | n.s. | * |
| Probe | 2.31 | 2.24 | n.s. | - | * | ||
| Portable | 1.88 | 3.03 | * | * | - | ||
| 1/TPC (kg/gGAE) | 0.023 | Sphere | 0.045 | 0.044 | - | n.s. | * |
| Probe | 0.044 | 0.043 | n.s. | - | * | ||
| Portable | 0.036 | 0.052 | * | * | - | ||
| logDPPH• (log % inhib./mg) | 0.106 | Sphere | 0.257 | 0.287 | - | n.s. | * |
| Probe | 0.282 | 0.267 | n.s. | - | * | ||
| Portable | 0.223 | 0.390 | * | * | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, S.; Jolayemi, O.S.; Giovenzana, V.; Tugnolo, A.; Squeo, G.; Conte, P.; De Bruno, A.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives. Foods 2021, 10, 1042. https://doi.org/10.3390/foods10051042
Grassi S, Jolayemi OS, Giovenzana V, Tugnolo A, Squeo G, Conte P, De Bruno A, Flamminii F, Casiraghi E, Alamprese C. Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives. Foods. 2021; 10(5):1042. https://doi.org/10.3390/foods10051042
Chicago/Turabian StyleGrassi, Silvia, Olusola Samuel Jolayemi, Valentina Giovenzana, Alessio Tugnolo, Giacomo Squeo, Paola Conte, Alessandra De Bruno, Federica Flamminii, Ernestina Casiraghi, and Cristina Alamprese. 2021. "Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives" Foods 10, no. 5: 1042. https://doi.org/10.3390/foods10051042
APA StyleGrassi, S., Jolayemi, O. S., Giovenzana, V., Tugnolo, A., Squeo, G., Conte, P., De Bruno, A., Flamminii, F., Casiraghi, E., & Alamprese, C. (2021). Near Infrared Spectroscopy as a Green Technology for the Quality Prediction of Intact Olives. Foods, 10(5), 1042. https://doi.org/10.3390/foods10051042