Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apple Juice and Pasteurized Apple Juice
2.2. Beetroots and Beetroot Juice
2.3. Beetroot and Apple Mixed Juice
2.4. Sugar Content
2.5. Dry Matter
2.6. pH
2.7. Colour Measurement
2.8. Betalains
2.9. Antioxidant Activity
2.10. Accelerated Shelf-Life Test
2.11. Data Elaboration and Statistics
3. Results
3.1. Effect of Processing
3.1.1. Colour
3.1.2. Betalains
3.1.3. Antioxidant Activity
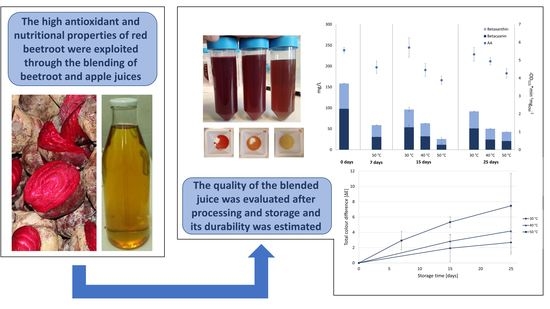
3.2. Preliminary Accelerated Shelf-Life Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakhare, K.S.; Sawate, A.R.; Kshirsagar, R.B.; Taur, A.T. Studies on evaluation of physical and chemical composition of beetroot (Beta vulgaris L.). Int. J. Chem. Stud. 2018, 7, 283–285. [Google Scholar]
- Karunaratne, D.N.; Pamunuwa, G.K. Food Additives; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties gro wn in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Guldiken, B.; Toydemir, G.; Nur Memis, K.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Wirleitner, B.; Schroecksn, K.; Schennach, H.; Fuchs, D. In Vitro effects of beet root juice on stimulated and unstimulated peripheral blood mononuclear cells. Am. J. Biochem. Biotechnol. 2005, 1, 180–185. [Google Scholar] [CrossRef]
- Kapadia, J.G.; Azuine, A.M.; Rao, G.S.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer Agents Med. Chem. 2012, 11, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Rao, G.S.; Ramachandran, C.; Iida, A.; Suzuki, N.; Tokuda, H. Synergistic cytotoxicity of red beetroot (Beta vulgaris L.) extract with doxorubicin in human pancreatic, breast and prostate cancer cell lines. J. Complement. Integr. Med. 2013, 10, 113–122. [Google Scholar] [CrossRef]
- Esquivel, P.B. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color, 1st ed.; Carle, R., Schweiggert, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–99. [Google Scholar]
- Porto, M.; Okina, V.; Pimentel, T.; Prudencio, S. Physicochemical stability, antioxidant activity, and acceptance of beet and orange mixed juice during refrigerated storage. Beverages 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef]
- Lee, E.J.; An, D.; Nguyen, C.T.T.; Patil, B.S.; Kim, J.; Yoo, K.S. Betalain and betaine composition of greenhouse- or field-produced beetroot (Beta vulgaris L.) and inhibition of HepG2 cell proliferation. J. Agric. Food Chem. 2014, 62, 1324–1331. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A.; Forrestre, T.; Galuska, D.A.; Herman, S.; James, W.P.T.; M’Buyamba Kabangu, J.R.; et al. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003. [Google Scholar] [CrossRef] [Green Version]
- Eurostat. Fruit and Vegetable Consumption Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Fruit_and_vegetable_consumption_statistics#General_overview (accessed on 3 March 2021).
- Fidelis, M.; Santos, J.S.; Coelho, A.L.K.; Rodionova, O.Y.; Pomerantsev, A.; Granato, D. Authentication of juices from antioxidant and chemical perspectives: A feasibility quality control study using chemometrics. Food Control 2017, 73, 796–805. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red beetroot. A potential source of natural additives for the meat industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Kazimierczak, R.E.; Jabłońska, P.; Rembiałkowska, E.W.A. Analysis of organic and conventional beetroot juice assortment in Warsaw shops and consumer evaluation of selected products. In Proceedings of the 4th ISOFAR Scientific Conference, Istanbul, Turkey, 13–15 October 2014. [Google Scholar]
- Bhardwaj, R.L.; Pandey, S. Juice blends-a way of utilization of under-utilized fruits, vegetables, and spices: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dambalkar, V.S.; Rudrawar, B.D.; Poojari, V.R. Study of Physico-Chemical Properties and Sensory Attributes of Beetroot-Orange RTS Drink. Int. J. Sci. Res. 2015, 4, 549–589. [Google Scholar]
- El-Dakak, A.; Youssef, M.; Abd El-Rahman, H. Evaluation of beetroot juice blends with carrot and apple juice as healthy beverage. Bull. Natl. Nutr. Inst. 2017, 48, 1–29. [Google Scholar] [CrossRef]
- Emelike, N.J.T.; Barber, L.I.; Ebere, C.O. Quality characteristics of beetroot juice treated with indigenous spices (lemon, ginger and ehuru). Int. J. Food Sci. Nutr. Eng. 2016, 6, 14–19. [Google Scholar] [CrossRef]
- Neelwarne, B. Red Beet Biotechnology: Food and Pharmaceutical Applications, 1st ed.; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Kathiravan, T.; Nadanasabapathi, S.; Kumar, R. Standardization of process condition in batch thermal pasteurization and its effect on antioxidant, pigment and microbial inactivation of Ready to Drink (RTD) beetroot (Beta vulgaris L.) juice. Int. Food Res. J. 2014, 21, 1305–1312. [Google Scholar]
- Venir, E.; Romano, G.; Hack, F.M. Nischenprodukt rohnensaft. Südtiroler Landwirt 2018, 16, 71–73. [Google Scholar]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Tobolková, B.; Polovka, M.; Daško, Ľ.; Belajová, E.; Durec, J. Evaluation of qualitative changes of apple-beetroot juice during long-term storage at different temperatures. J. Food Meas. Charact. 2020, 14, 3381–3388. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Alimentarius: International Food Standards. Code of Hygienic Practice for Low and Acidified Low Acid Canned Foods (CAC/RCP 23-1979). Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/codes-of-practice/en/ (accessed on 3 March 2021).
- Horwitz, W. Sugars and Sugar Products. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Manzocco, L.; Anese, M.; Nicoli, M.C. Antioxidant properties of tea extracts as affected by processing. LWT Food Sci. Technol. 1998, 31, 694–698. [Google Scholar] [CrossRef]
- Man, D.C.M. Shelf Life, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Saftner, R.A.; Daie, J.; Wyse, R.E. Sucrose uptake and compartmentation in sugar beet taproot tissue. Plant Physiol. 1983, 72, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Zhu, Z.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Luo, D.; Yu, L.; Westland, S.; Mahon, N. The influence of colour and image on consumer purchase intentions of convenience food. J. Int. Colour Assoc. 2019, 24, 11–23. [Google Scholar]
- Plasek, B.; Temesi, Á. Factors that influence the perceived healthiness of food—Review. Nutrients 2020, 12, 1881. [Google Scholar] [CrossRef]
- Grunert, K.G. The meaning of colours in nutrition labelling in the context of expert and consumer criteria of evaluating food product healthfulness. J. Health Psychol. 2015, 20, 907–920. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, 491–499. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Pedreño, M.A.; Escribano, J. Studying the oxidation and the antiradical activity of betalain from beetroot. J. Biol. Educ. 2000, 35, 49–51. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colorants; Hendry, G.A.F., Houghton, J.D., Eds.; Springer: Boston, MA, USA, 1996. [Google Scholar]
- Veberic, R.; Stampar, F.; Schmitzer, V.; Cunja, V.; Zupan, A.; Koron, D.; Mikulic-Petkovsek, M. Changes in the contents of anthocyanins and other compounds in blackberry fruits due to freezing and long-term frozen storage. J. Agric. Food Chem. 2014, 62, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Institute of Food Science and Technology (IFST). Shelf Life of Foods: Guidelines for its Determination and Prediction; Institute of Food Science and Technology: London, UK, 1993. [Google Scholar]
- Chandran, J.; Nisha, P.; Singhal, R.S.; Pandit, A.B. Degradation of colour in beetroot (Beta vulgaris L.): A kinetics study. J. Food Sci. Technol. 2014, 51, 2678–2684. [Google Scholar] [CrossRef] [Green Version]
- Kathiravan, T.; Nadanasabapathi, S.; Kumar, R. Pigments and antioxidant activity of optimized Ready-to-Drink (RTD) Beetroot (Beta vulgaris L.)-passion fruit (Passiflora edulis var. flavicarpa) juice blend. Croat. J. Food Sci. Technol. 2015, 7, 9–21. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- De Gaulejac, N.S.C.; Vivas, N.; De Freitas, V.; Bourgeois, G. The influence of various phenolic compounds on scavenging activity assessed by an enzymatic method. J. Sci. Food Agric. 1999, 79, 1081–1090. [Google Scholar] [CrossRef]
- Pinelo, M.; Manzocco, L.; Nuñez, M.J.; Nicoli, M.C. Solvent effect on quercetin antioxidant capacity. Food Chem. 2004, 88, 201–207. [Google Scholar] [CrossRef]








| Sample Code | Sample Name | Description |
|---|---|---|
| AJ | Apple juice | Cloudy apple juice purchased on the market |
| PAJ | Pasteurized apple juice | Apple juice (AJ) subjected to pasteurization |
| FBJ | Fresh beetroot juice | Raw beetroot juice obtained through a household juice extractor |
| BJ | Beetroot juice | Beetroot juice obtained by means of pilot-scale turboextractor |
| PBJ | Pasteurized beetroot juice | Beetroot juice (BJ) subjected to pasteurization |
| MIX | Beetroot and apple juice blend | Blended juice obtained by diluting beetroot juice (BJ; 15%) with apple juice (AJ; 85%) |
| PMIX | Pasteurized beetroot and apple juice blend | Beetroot and apple juice blend subjected to pasteurization |
| Comparison | Processing Step under Investigation |
|---|---|
| BJ-FBJ | Effect of processing-extraction |
| FBJ-PBJ | Effect of pasteurization on the quality of beetroot juice |
| AJ-PAJ | Effect of pasteurization of apple juice |
| MIX-PMIX | Effect of pasteurization of the beetroot and apple juice blend |
| AJ/BJ-MIX | Effect of addition of apple juice to beetroot juice |
| Sample Code | pH | Sugar Content (°Brix) |
|---|---|---|
| AJ | 3.76 ± 0.01 | 12.77 ± 0.05 |
| PAJ | 3.92 ± 0.06 | 13.15 ± 1.13 |
| FBJ | 6.45 ± 0.03 | 15.72 ± 0.04 |
| BJ | 6.31 ± 0.01 | 10.37 ± 0.05 |
| PBJ | 6.10 ± 0.01 | 9.87 ± 0.05 |
| MIX | 4.16 ± 0.06 | 12.27 ± 0.08 |
| PMIX | 4.22 ± 0.06 | 12.15 ± 0.18 |
| Sample Code | CIE Colour Values | ||
|---|---|---|---|
| L* | a* | b* | |
| FBJ | 16.38 ± 0.17 | 4.78 ± 0.48 | 0.60 ± 0.11 |
| BJ | 16.12 ± 0.13 | 3.76 ± 0.58 | 0.63 ± 0.13 |
| PBJ | 16.75 ± 1.08 | 4.30 ± 0.35 | 1.09 ± 0.16 |
| MIX | 16.27 ± 0.07 | 3.81 ± 0.09 | 0.40 ± 0.10 |
| PMIX | 17.23 ± 0.22 | 8.96 ± 1.29 | 1.63 ± 0.36 |
| Sample Code | ∆E |
|---|---|
| FBJ-BJ | 1.07 ± 0.30 |
| BJ-MIX | 0.53 ± 0.35 |
| MIX-PMIX | 5.38 ± 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, F.; Pünsch, M.; Venir, E. Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice. Foods 2021, 10, 1052. https://doi.org/10.3390/foods10051052
Bianchi F, Pünsch M, Venir E. Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice. Foods. 2021; 10(5):1052. https://doi.org/10.3390/foods10051052
Chicago/Turabian StyleBianchi, Flavia, Marina Pünsch, and Elena Venir. 2021. "Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice" Foods 10, no. 5: 1052. https://doi.org/10.3390/foods10051052
APA StyleBianchi, F., Pünsch, M., & Venir, E. (2021). Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice. Foods, 10(5), 1052. https://doi.org/10.3390/foods10051052