Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation
Abstract
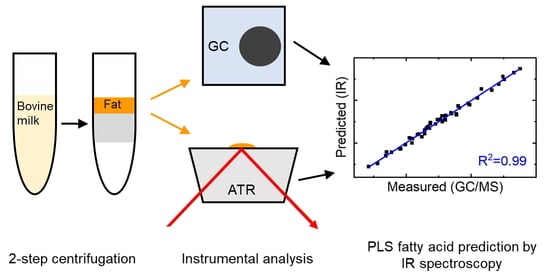
:1. Introduction
2. Materials and Methods
2.1. Milk Samples
2.2. Fat Separation
2.3. GC/MS Analysis
2.4. FT–IR Measurements
2.5. Data Analysis
3. Results and Discussion
3.1. Comparison of IR Spectra of Whole Milk and Separated Milk Fat Fraction
3.2. Predicting Fatty Acid Content by Mid-IR Spectroscopy
3.2.1. Partial Least Squares Analysis
3.2.2. Selection of Wavenumber Range Based on Selectivity Ratio
3.2.3. Evaluation of Covariation Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Paris, France, 2020. [Google Scholar] [CrossRef]
- Jenness, R.; Wong, N.P.; Marth, E.H.; Keeney, M. Fundamentals of Dairy Chemistry; Springer: New York City, NY, USA, 1988. [Google Scholar] [CrossRef]
- Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmquist, D.L.; Denise Beaulieu, A.; Barbano, D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- German, J.B.; Gibson, R.A.; Krauss, R.M.; Nestel, P.; Lamarche, B.; van Staveren, W.A.; Steijns, J.M.; de Groot, L.C.; Lock, A.L.; Destaillats, F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur. J. Nutr. 2009, 48, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Brandstetter, M.; Lendl, B. Quantum cascade lasers (QCLs) in biomedical spectroscopy. Chem. Soc. Rev. 2017, 46, 5903–5924. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited review: Mid-infrared spectroscopy as phenotyping tool for milk traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef]
- Voort, F.R.V.D. Evaluation of Milkoscan 104 Infrared Milk Analyzer. J. AOAC Int. 2020, 63, 973–980. [Google Scholar] [CrossRef]
- Kuligowski, J.; Schwaighofer, A.; Alcaraz, M.R.; Quintas, G.; Mayer, H.; Vento, M.; Lendl, B. External cavity-quantum cascade laser (EC-QCL) spectroscopy for protein analysis in bovine milk. Anal. Chim. Acta 2017, 963, 99–105. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Kuligowski, J.; Quintas, G.; Mayer, H.K.; Lendl, B. Fast quantification of bovine milk proteins employing external cavity-quantum cascade laser spectroscopy. Food Chem. 2018, 252, 22–27. [Google Scholar] [CrossRef]
- Montemurro, M.; Schwaighofer, A.; Schmidt, A.; Culzoni, M.J.; Mayer, H.K.; Lendl, B. High-throughput quantitation of bovine milk proteins and discrimination of commercial milk types by external cavity-quantum cascade laser spectroscopy and chemometrics. Analyst 2019, 144, 5571–5579. [Google Scholar] [CrossRef] [Green Version]
- Schwaighofer, A.; Alcaráz, M.R.; Kuligowski, J.; Lendl, B. Recent advancements of EC-QCL based mid-IR transmission spectroscopy of proteins and application to analysis of bovine milk. Biomed. Spectrosc. Imaging 2018, 7, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Ramer, G.; Lendl, B. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy. In Encyclopedia of Analytical Chemistry; Meyers, R., RAMTECH Inc., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Sherazi, S.T.H.; Talpur, M.Y.; Mahesar, S.A.; Kandhro, A.A.; Arain, S. Main fatty acid classes in vegetable oils by SB-ATR-Fourier transform infrared (FTIR) spectroscopy. Talanta 2009, 80, 600–606. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Mossoba, M.M.; Chung, J.K.; Haile, E.A.; Srigley, C.T. Rapid Prediction of Fatty Acid Content in Marine Oil Omega-3 Dietary Supplements Using a Portable Fourier Transform Infrared (FTIR) Device and Partial Least-Squares Regression (PLSR) Analysis. J. Agric. Food Chem. 2017, 65, 224–233. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Lognay, G.; Veselko, D.; Marlier, M.; Bertozzi, C.; Mayeres, P.; Gengler, N. Estimating Fatty Acid Content in Cow Milk Using Mid-Infrared Spectrometry. J. Dairy Sci. 2006, 89, 3690–3695. [Google Scholar] [CrossRef] [Green Version]
- Soyeurt, H.; Dehareng, F.; Mayeres, P.; Bertozzi, C.; Gengler, N. Variation of Δ9-Desaturase Activity in Dairy Cattle. J. Dairy Sci. 2008, 91, 3211–3224. [Google Scholar] [CrossRef] [Green Version]
- Rutten, M.J.M.; Bovenhuis, H.; Hettinga, K.; Valenberg, H.J.F.; Arendonk, J. Predicting bovine milk fat composition using infrared spectroscopy based on milk samples collected in winter and summer. J. Dairy Sci. 2009, 92, 6202–6209. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Mele, M.; Secchiari, P.; Bittante, G. Effectiveness of mid-infrared spectroscopy to predict fatty acid composition of Brown Swiss bovine milk. Animal 2011, 5, 1653–1658. [Google Scholar] [CrossRef] [Green Version]
- Ferrand, M.; Huquet, B.; Barbey, S.; Barillet, F.; Lahalle, F.; Larroque, H.; Leray, O.; Trommenschlager, J.M.; Brochard, M. Determination of fatty acid profile in cow’s milk using mid-infrared spectrometry: Interest of applying a variable selection by genetic algorithms before a PLS regression. Chemom. Intell. Lab. Syst. 2011, 106, 183–189. [Google Scholar] [CrossRef]
- Eijndhoven, M.H.T.; Soyeurt, h.; Dehareng, F.; Calus, M. Validation of fatty acid predictions in milk using mid-infrared spectrometry across cattle breeds. Animal 2012, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.; Coffey, M.; Dardenne, P. Mid-infrared prediction of bovine milk fatty acids across multiple breeds, production systems, and countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef] [Green Version]
- Eskildsen, C.; Rasmussen, M.; Engelsen, S.; Larsen, L.; Poulsen, N.A.; Skov, T. Quantification of individual fatty acids in bovine milk by infrared spectroscopy and chemometrics: Understanding predictions of highly collinear reference variables. J. Dairy Sci. 2014, 97, 7940–7951. [Google Scholar] [CrossRef] [Green Version]
- Afseth, N.K.; Martens, H.; Randby, Å.; Gidskehaug, L.; Narum, B.; Jørgensen, K.; Lien, S.; Kohler, A. Predicting the Fatty Acid Composition of Milk: A Comparison of Two Fourier Transform Infrared Sampling Techniques. Appl. Spectrosc. 2010, 64, 700–707. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Lunder, T.L. Simplified Procedure for Determining Fat and Total Solids by Mojonnier Method. J. Dairy Sci. 1971, 54, 737–739. [Google Scholar] [CrossRef]
- ISO. Milk and Milk Products—Extraction Methods for Lipids and Liposoluble Compounds; ISO 1415:2001; ISO Publications: Geneva, Switzerland, 2001. [Google Scholar]
- Stefanov, I.; Vlaeminck, B.; Fievez, V. A novel procedure for routine milk fat extraction based on dichloromethane. J. Food Compos. Anal. 2010, 23, 852–855. [Google Scholar] [CrossRef]
- Feng, S.; Lock, A.L.; Garnsworthy, P.C. Technical Note: A Rapid Lipid Separation Method for Determining Fatty Acid Composition of Milk. J. Dairy Sci. 2004, 87, 3785–3788. [Google Scholar] [CrossRef] [Green Version]
- Luna, P.; Juárez, M.; Fuente, M.A. Validation of a Rapid Milk Fat Separation Method to Determine the Fatty Acid Profile by Gas Chromatography. J. Dairy Sci. 2005, 88, 3377–3381. [Google Scholar] [CrossRef] [Green Version]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Kaylegian, K.E.; Lynch, J.M.; Fleming, J.R.; Barbano, D. Influence of fatty acid chain length and unsaturation on mid-infrared milk analysis. J. Dairy Sci. 2009, 92, 2485–2501. [Google Scholar] [CrossRef]
- Baker, M.J.; Hussain, S.R.; Lovergne, L.; Untereiner, V.; Hughes, C.; Lukaszewski, R.A.; Thiéfin, G.; Sockalingum, G.D. Developing and understanding biofluid vibrational spectroscopy: A critical review. Chem. Soc. Rev. 2016, 45, 1803–1818. [Google Scholar] [CrossRef] [Green Version]
- Pivonka, D.E.; Kirkland, K.M. Research Strategy for the HPLC/FT-IR Analysis of Drug Metabolites. Appl. Spectrosc. 1997, 51, 866–873. [Google Scholar] [CrossRef]
- Kohler, A.; Afseth, N.; Jørgensen, K.; Randby, Å.; Martens, H. Quality Analysis of Milk by Vibrational Spectroscopy. In Handbook of Vibrational Spectroscopy; Jim, C., Peter, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.-F.; Genot, C. Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Lendl, B. Chapter 3—Quantum cascade laser-based infrared transmission spectroscopy of proteins in solution. In Vibrational Spectroscopy in Protein Research; Ozaki, Y., Baranska, M., Lednev, I.K., Wood, B.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 59–88. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Pahlow, S.; Hübner, U.; Popp, J. CaF2: An Ideal Substrate Material for Infrared Spectroscopy? Anal. Chem. 2020, 92, 9024–9031. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Soulat, J.; Andueza, D.; Graulet, B.; Girard, C.L.; Labonne, C.; Aït-Kaddour, A.; Martin, B.; Ferlay, A. Comparison of the Potential Abilities of Three Spectroscopy Methods: Near-Infrared, Mid-Infrared, and Molecular Fluorescence, to Predict Carotenoid, Vitamin and Fatty Acid Contents in Cow Milk. Foods 2020, 9, 592. [Google Scholar] [CrossRef]
- Kvalheim, O.M. Variable importance: Comparison of selectivity ratio and significance multivariate correlation for interpretation of latent-variable regression models. J. Chemom. 2020, 34, e3211. [Google Scholar] [CrossRef] [Green Version]
- V.D. dos Santos, A.C.; Heydenreich, R.; Derntl, C.; Mach-Aigner, A.R.; Mach, R.L.; Ramer, G.; Lendl, B. Nanoscale Infrared Spectroscopy and Chemometrics Enable Detection of Intracellular Protein Distribution. Anal. Chem. 2020, 92, 15719–15725. [Google Scholar] [CrossRef]
- Christy, A.A.; Egeberg, P.K. Quantitative determination of saturated and unsaturated fatty acids in edible oils by infrared spectroscopy and chemometrics. Chemom. Intell. Lab. Syst. 2006, 82, 130–136. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Valcarcel, M.; Ayora-Canada, M.J. Two-dimensional correlation spectroscopy and multivariate curve resolution for the study of lipid oxidation in edible oils monitored by FTIR and FT-Raman spectroscopy. Anal. Chim. Acta 2007, 593, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. The effects of chain length on the infrared spectra of fatty acids and methyl esters. Can. J. Chem. 1962, 40, 321–333. [Google Scholar] [CrossRef]
- Rice, J.A. Mathematical Statistics and Data Analysis, 3rd ed.; Crockett, C., Ed.; Thompson/Brooks/Cole: Belmont, CA, USA, 2007. [Google Scholar]




| Wavenumber/cm−1 | Detectable in Whole Milk * | Group | Mode of Vibration | Functional Group |
|---|---|---|---|---|
| 3005 | no | C–H | sym. stretch | -C=CH- (cis) |
| 2953 | yes | C–H | asym. stretch | -CH3 (aliphatic) |
| 2922 | yes | C–H | asym. stretch | -CH2- (aliphatic) |
| 2853 | yes | C–H | sym. stretch | -CH2- (aliphatic) |
| 1743 | yes | C=O | stretch | C=O ester |
| 1655 | no | C=C | stretch | C=C (unsaturated) |
| 1462 | overlapping | C–H | scissoring | -CH2- (aliphatic) |
| 1377 | overlapping | C–H | sym. deformation | -CH3 (aliphatic) |
| 1238 | overlapping | C–H | out-of-plane bend | -CH2- (aliphatic) |
| 1162 | overlapping | C–O | stretch | C-O ester |
| 966 | no | C–H | out-of-plane bend | -C=CH- (trans) |
| 722 | no | C–H | rocking | -CH2- (aliphatic) |
| g/100 g Fat | ||||||||
|---|---|---|---|---|---|---|---|---|
| Full Dataset (n = 45) | Split Dataset (n= 30/15) | |||||||
| Fatty Acid | LVs | Range | RMSEC | RMSECV | R2 | R2CV | RMSEP | R2P |
| SAT | 8 | 61.6–74.5 | 0.27 | 0.66 | 0.99 | 0.94 | 0.8 | 0.95 |
| MONO | 8 | 19.8–30.3 | 0.28 | 0.57 | 0.99 | 0.95 | 0.74 | 0.94 |
| PUFA | 3 | 2.2–4.2 | 0.20 | 0.24 | 0.73 | 0.61 | 0.28 | 0.27 |
| UNSAT | 8 | 22.1–33.8 | 0.28 | 0.58 | 0.99 | 0.96 | 0.74 | 0.95 |
| SCFA | 7 | 14.2–21.0 | 0.45 | 0.78 | 0.87 | 0.64 | 0.67 | 0.83 |
| MCFA | 7 | 38.1–56.0 | 0.57 | 0.96 | 0.98 | 0.95 | 0.85 | 0.97 |
| LCFA | 7 | 26.4–47.7 | 0.43 | 0.76 | 0.99 | 0.98 | 0.65 | 0.99 |
| C4:0 | 6 | 5.4–8.8 | 0.27 | 0.42 | 0.87 | 0.72 | 0.49 | 0.62 |
| C6:0 | 5 | 3.1–5.4 | 0.20 | 0.31 | 0.72 | 0.38 | 0.24 | 0.71 |
| C8:0 | 5 | 1.5–3.2 | 0.12 | 0.16 | 0.81 | 0.64 | 0.11 | 0.88 |
| C10:0 | 7 | 2.1–4.9 | 0.05 | 0.11 | 0.99 | 0.97 | 0.10 | 0.99 |
| C12:0 | 5 | 2.0–5.6 | 0.09 | 0.16 | 0.99 | 0.96 | 0.19 | 0.97 |
| C14:0 | 7 | 7.4–13.3 | 0.20 | 0.49 | 0.97 | 0.85 | 0.48 | 0.88 |
| C16:0 | 8 | 21.1–35.1 | 0.40 | 1.05 | 0.98 | 0.85 | 1.4 | 0.81 |
| C16:1cis | 4 | 1.2–3.9 | 0.28 | 0.41 | 0.73 | 0.44 | 0.44 | 0.39 |
| C18:0 | 5 | 5.6–14.6 | 0.38 | 0.57 | 0.97 | 0.93 | 0.63 | 0.93 |
| C18:1cis | 8 | 14.9–27.2 | 0.22 | 0.74 | 0.99 | 0.92 | 0.77 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhgar, C.K.; Nürnberger, V.; Nadvornik, M.; Velik, M.; Schwaighofer, A.; Rosenberg, E.; Lendl, B. Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation. Foods 2021, 10, 1054. https://doi.org/10.3390/foods10051054
Akhgar CK, Nürnberger V, Nadvornik M, Velik M, Schwaighofer A, Rosenberg E, Lendl B. Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation. Foods. 2021; 10(5):1054. https://doi.org/10.3390/foods10051054
Chicago/Turabian StyleAkhgar, Christopher Karim, Vanessa Nürnberger, Marlene Nadvornik, Margit Velik, Andreas Schwaighofer, Erwin Rosenberg, and Bernhard Lendl. 2021. "Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation" Foods 10, no. 5: 1054. https://doi.org/10.3390/foods10051054
APA StyleAkhgar, C. K., Nürnberger, V., Nadvornik, M., Velik, M., Schwaighofer, A., Rosenberg, E., & Lendl, B. (2021). Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation. Foods, 10(5), 1054. https://doi.org/10.3390/foods10051054