Ca and Mg Concentrations in Spices and Growth of Commonly Sporulated and Non-Sporulated Food-Borne Microorganisms According to Marketing Systems
Abstract
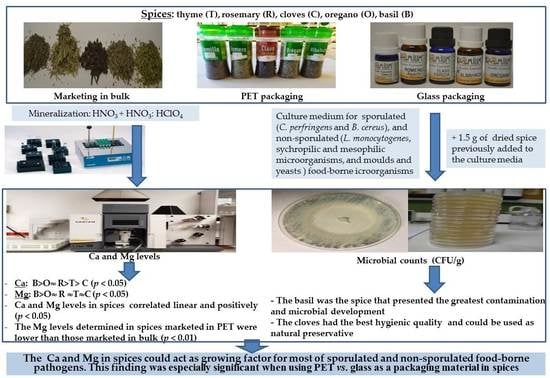
:1. Introduction
2. Materials and Methods
2.1. Sampling of Dried Spices
2.2. Ca and Mg Analysis in Spices
2.3. Microbiological Analysis Methods
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferriccioni, N.; Mateucci, R.; Zangrando, A.; Santana, S.; Campos, C.A. Effect of decontamination treatment on the quality of dehydrated thyme, coriander, and mustard. Food Sci. Technol. Int. 2019, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, J.M.; Neil, K.P.; Parish, M.; Gieraltowski, L.; Gould, L.H.; Gombas, K.L. Foodborne illness outbreaks from microbial contaminants in spices, 1973–2010. Food Microbiol. 2013, 36, 456–464. [Google Scholar] [CrossRef]
- García-Galdeano, J.M.; Villalón-Mir, M.; Medina-Martínez, J.; Vázquez-Foronda, L.M.; Zamora-Bustillos, J.G.; Agil, A.; Moor-Davie, S.M.F.; Navarro-Alarcón, M. Zn, Cu, and Fe Concentrations in Dehydrated Herbs (Thyme, Rosemary, Cloves, Oregano, and Basil) and the Correlation with the Microbial Counts of Listeria monocytogenes and Other Foodborne Pathogens. Foods 2020, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.; Quevedo, C.; Graça, A.; Quintas, C. Hygienic quality of dehydrated aromatic herbs marketed in Southern Portugal. AIMS Agric. Food 2020, 5, 46–53. [Google Scholar] [CrossRef]
- Batt, C.A.; Tortorello, M.L. (Eds.) Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: London, UK, 2014; Volume 1. [Google Scholar]
- Little, C.L.; Omotoye, R.; Mitchell, R.T. The microbiological quality of ready-to-eat foods with added spices. Int. J. Environ. Health Res. 2003, 13, 31–42. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Goleen, D.A. (Eds.) Modern Food Microbiology, 7th ed.; Springer Science and Business Media: New York, NY, USA, 2005. [Google Scholar]
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its preservatives, additives and applications. Int. J. Chem. Biol. Sci. 2012, 1, 36–47. [Google Scholar]
- Bor, T.; Gyawali, R.; Ibrahim, S.A. Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium. Foods 2016, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Tajkarimi, M.; Ibrahim, S.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control. 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Silva, N.; Barbosa, L.; Seito, L.; Junior, A.F. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat. Prod. Res. 2012, 26, 1510–1514. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Ali, H.I.; Al-Younis, Z.K. The effect of adding thyme extacts on microbiological, chemical and sensory characteristics of yogurt. J. Pure Appl. Microbiol. 2020, 14, 1367–1376. [Google Scholar] [CrossRef]
- Gekenidis, M.-T.; Gossin, D.; Schmelcher, M.; Schöner, U.; Remus-Emsermann, M.N.P.; Drissner, D. Dynamics of culturable mesophilic bacterial communities of three fresh herbs and their production environment. J. Appl. Microbiol. 2017, 123, 916–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drissner, D.; Zuercher, U. Safety of Food and Beverages: Fruits and Vegetables. Encycl. Food Saf. 2014, 3, 253–259. [Google Scholar] [CrossRef]
- Sospedra, I.; Soriano, J.M.; Mañes, J. Assessment of the Microbiological Safety of Dried Spices and Herbs Commercialized in Spain. Plant Foods Hum. Nutr. 2010, 65, 364–368. [Google Scholar] [CrossRef]
- Food and Drugs Administration (FDA). Draft Risk Profile: Pathogens and Filth in Spices 2013. Available online: https://www.fda.gov/media/86724/download (accessed on 1 January 2021).
- Wen, Q.; McClane, B.A. Detection of Enterotoxigenic Clostridium perfringens Type A Isolates in American Retail Foods. Appl. Environ. Microbiol. 2004, 70, 2685–2691. [Google Scholar] [CrossRef] [Green Version]
- Vizzini, P.; Beltrame, E.; Zanet, V.; Vidic, J.; Manzano, M. Development and evaluation of qPCR detection method and Zn-MgO/alginate active packaging for controlling Listeria monocytogenes contamination in cold-smoked salmon. Foods 2020, 9, 1353. [Google Scholar] [CrossRef]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6, 907. [Google Scholar] [CrossRef] [Green Version]
- López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R.; Ocio, M.J. Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int. J. Food Microbiol. 2008, 126, 65–70. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Fu, L. MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef]
- Kumar, M.; Srivastava, S. Effect of calcium and magnesium on the antimicrobial action of enterocin LR/6 produced by Enterococcus faecium LR/6. Int. J. Antimicrob. Agents 2011, 37, 572–575. [Google Scholar] [CrossRef]
- Lenz, C.A.; Vogel, R.F. Effect of sporulation medium and its divalent cation content on the heat and high pressure resistance of Clostridium botulinum type E spores. Food Microbiol. 2014, 44, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Jo, J.; Ryu, S.; Kim, K.-P. Bacteriophages BCP1-1 and BCP8-2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiol. 2012, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, M.; Otsuka, K.; Miyake, M. Nitrate salts suppress sporulation and production of enterotoxin in Clostridium perfringens strain NCTC8239. Microbiol. Immunol. 2016, 60, 657–668. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J. Ángel Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO 7932:2005). Microbiology of Food and Animal Feeding stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus Cereus—Colony-Count Technique at 30 °C; ISO Publishers: Geneve, Italy, 2005. [Google Scholar]
- International Organization for Standardization (ISO 7937:2005). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Clostridium Perfringens—Colony-Count Technique; ISO Publishers: Geneve, Italy, 2005. [Google Scholar]
- International Organization for Standardization (ISO 4833:2003). Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Microorganisms-Colony Count Technique at 30 °C; ISO Publishers: Geneve, Italy, 2003. [Google Scholar]
- International Organization for Standardization (ISO 21527-2:2008). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0,95; ISO Publishers: Geneve, Italy, 2008. [Google Scholar]
- International Organization for Standardization (ISO 11290:2017). Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and Listeria spp”. Part 1: Detection Method and Part 2: Enumeration Method; ISO Publishers: Geneve, Italy, 2017. [Google Scholar]
- Özcan, M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2008, 106, 852–858. [Google Scholar] [CrossRef]
- Kara, D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Zengin, M.; Özcan, M.M.; Çetin, Ü.; Gezgin, S. Mineral contents of some aromatic plants, their growth soils and infusions. J. Sci. Food Agric. 2008, 88, 581–589. [Google Scholar] [CrossRef]
- Potorti, A.G.; Bua, G.D.; Lo Turco, V.; Ben Tekaya, A.B.; Beltifa, A.; Ben Mansour, H.; Dugo, G.; Di Bella, G. Major, minor and trance element concentrations in spices and aromatic herbs from Sicily (Italy) and Mahdia (Tunisia) by ICP-MS and multivariate analysis. Food Chem. 2020, 313, 126094. [Google Scholar] [CrossRef]
- Sabina, R.-O.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in aromatic plant species from the abandoned Rosalgar Mine and their potential risk to human health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar] [CrossRef]
- Obiajunwa, E.I.; Adebajo, A.C.; Omobuwajo, O.R. Essential and trace element contents of some Nigerian medicinal plants. J. Radioanal. Nucl. Chem. 2002, 252, 473–476. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization Statistic Database 2013. Available online: http://faostat3.fao.org/faostat-gateway/go/to/home/F (accessed on 25 January 2021).
- Institute of Medicine. Food and Nutrition Board. In Dietary Reference Intakes (DRIs) for Elements; The National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Jodral-Segado, A.M.; Navarro-Alarcón, M.; López-García de la Serrana, H.; López-Martínez, M.C. Magnesium and calcium contents in foods from SE Spain: Influencing factors and estimation of daily dietary intakes. Sci. Total Environ. 2003, 312, 47–58. [Google Scholar] [CrossRef]
- Drake, S.; Elfving, D.; Visser, D.; Drake, S. Quality of modified atmosphere packaged “bartlett” pears as influenced by time and type of storage. J. Food Process. Preserv. 2005, 28, 348–358. [Google Scholar] [CrossRef]
- Brody, A.L. Modified Atmosphere Packaging. In Encyclopedia of Agricultural, Food, and Biological Engineering; Heldman, D.R., Moraru, C., Eds.; Taylor & Francis: New York, NY, USA, 2003; pp. 666–670. [Google Scholar]
- Miranda, G.; Berna, À.; González, R.; Mulet, A. The storage of dried apricots: The effect of packaging and temperature on the changes of texture and moisture. J. Food Process. Preserv. 2014, 38, 565–572. [Google Scholar] [CrossRef]
- Balzarotti, S.; Maviglia, B.; Biassoni, F.; Ciceri, M. Glass vs. Plastic: Affective Judgments of Food Packages After Visual and Haptic Exploration. Procedia Manuf. 2015, 3, 2251–2258. [Google Scholar] [CrossRef]

| Spice | Ca ± SEM | Mg ± SEM | Reference |
|---|---|---|---|
| Thyme | 9.58 ± 2.66 | 1.53 ± 0.144 | [34] |
| Thyme | 7.76 ± 6.8 | 2.11 ± 6.2 | [35] |
| Thyme | 3.15 ± 0.12 | 0.47 ± 0.01 | [36] |
| Thyme | 834 ± 70.3 | 380 ± 73.3 | [37] |
| Thyme | 823 ± 135 | 134 ± 32.2 | [37] |
| Thyme | 16.5 ± 1.21 | 4.55 ± 0.207 | Present study |
| Rosemary | 8.60 ± 1.91 | 2.41 ± 0.264 | [34] |
| Rosemary | 306 ± 43.8 | 36.8 ± 3.40 | [37] |
| Rosemary | 297 ± 34.5 | 120 ± 17.8 | [37] |
| Rosemary | 21.9 ± 0.846 | 4.20 ± 0.457 | Present study |
| Cloves | 6.50 ± 0.789 | 2.89 ± 0.122 | [34] |
| Cloves | 5.58 ± 0.315 | 4.59 ± 0.356 | Present study |
| Oregano | 12.7 ± 2.19 | 3.09 ± 1.11 | [38] |
| Oregano | 422 ± 69.5 | 55.0 ± 6.10 | [37] |
| Oregano | 793 ± 62.3 | 355 ± 20.0 | [37] |
| Oregano | 20.7 ± 1.16 | 4.90 ± 0.349 | Present study |
| Basil | 26.7 | - | [39] |
| Basil | 5.56 ± 1.71 | 0.53 ± 0.01 | [36] |
| Basil | 16.5 ± 2.96 | 3.13 ± 0.443 | [34] |
| Basil | 32.7 ± 1.17 | 12.5 ± 0.561 | Present study |
| Marketing System | Ca ± SEM | Mg ± SEM |
|---|---|---|
| PET | 21.3 ± 2.18 a | 5.10 ± 0.494 a |
| Glass | 18.9 ± 1.91 a | 6.34 ± 0.787 a,b |
| Bulk | 17.3 ± 1.32 a | 6.53 ± 0.517 b |
| Microorganisms | Ca | Mg | ||
|---|---|---|---|---|
| ra | p | ra | p | |
| Sporulated microorganisms | ||||
| C. perfringens | 0.385 | 0.001 | 0.579 | 0.001 |
| B. cereus | 0.182 | 0.067 | 0.303 | 0.003 |
| Non-sporulated microorganisms | ||||
| L. monocytogenes | 0.265 | 0.007 | 0.198 | 0.054 |
| Psychrophilic microorganisms | 0.245 | 0.013 | 0.192 | 0.063 |
| Mesophilic microorganisms | 0.387 | 0.001 | 0.318 | 0.002 |
| Molds and yeasts | 0.405 | 0.001 | 0.024 | 0.817 |
| Microorganisms | Ca | Mg | ||||
|---|---|---|---|---|---|---|
| Bulk | PET | Glass | Bulk | PET | Glass | |
| Sporulated microroganismis | ||||||
| C. perfringens | 0.385 a (0.012) | 0.818 a (0.000) | 0.724 a (0.000) | 0.306 a (0.039) | 0.478 a (0.028) | 0.414 a (0.013) |
| B. cereus | 0.345 a (0.025) | 0.764 a (0.000) | 0.038 (0.837) | 0.174 (0.247) | 0.450 a (0.041) | −0.123 (0.480) |
| Non-sporulated microorganisms | ||||||
| L. monocytogenes | 0.289 (0.063) | 0.676 a (0.001) | −0.174 (0.341) | 0.163 (0.280) | 0.669 a (0.001) | −0.076 (0.683) |
| Psychrophilic microorganisms | 0.249 (0.111) | 0.654 a (0.001) | 0.376 a (0.034) | 0.643 a (0.000) | 0.330 (0.145) | 0.257 (0.136) |
| Mesophilic microorganisms | 0.352 a (0.022) | 0.132 (0.569) | 0.477 a (0.006) | 0.228 (0.128) | 0.470 a (0.032) | 0.044 (0.800) |
| Molds and yeasts | 0.232 (0.139) | 0.679 a (0.001) | −0.092 (0.617) | 0.353 a (0.016) | 0.685 a (0.001) | 0.291 a (0.000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galdeano, J.M.; Villalón-Mir, M.; Medina-Martínez, J.; Fonseca-Moor-Davie, S.M.; Zamora-Bustillos, J.G.; Vázquez-Foronda, L.M.; Agil, A.; Navarro-Alarcón, M. Ca and Mg Concentrations in Spices and Growth of Commonly Sporulated and Non-Sporulated Food-Borne Microorganisms According to Marketing Systems. Foods 2021, 10, 1122. https://doi.org/10.3390/foods10051122
García-Galdeano JM, Villalón-Mir M, Medina-Martínez J, Fonseca-Moor-Davie SM, Zamora-Bustillos JG, Vázquez-Foronda LM, Agil A, Navarro-Alarcón M. Ca and Mg Concentrations in Spices and Growth of Commonly Sporulated and Non-Sporulated Food-Borne Microorganisms According to Marketing Systems. Foods. 2021; 10(5):1122. https://doi.org/10.3390/foods10051122
Chicago/Turabian StyleGarcía-Galdeano, José María, Marina Villalón-Mir, José Medina-Martínez, Sofía María Fonseca-Moor-Davie, Jessandra Gabriela Zamora-Bustillos, Lydia María Vázquez-Foronda, Ahmad Agil, and Miguel Navarro-Alarcón. 2021. "Ca and Mg Concentrations in Spices and Growth of Commonly Sporulated and Non-Sporulated Food-Borne Microorganisms According to Marketing Systems" Foods 10, no. 5: 1122. https://doi.org/10.3390/foods10051122
APA StyleGarcía-Galdeano, J. M., Villalón-Mir, M., Medina-Martínez, J., Fonseca-Moor-Davie, S. M., Zamora-Bustillos, J. G., Vázquez-Foronda, L. M., Agil, A., & Navarro-Alarcón, M. (2021). Ca and Mg Concentrations in Spices and Growth of Commonly Sporulated and Non-Sporulated Food-Borne Microorganisms According to Marketing Systems. Foods, 10(5), 1122. https://doi.org/10.3390/foods10051122