Evaluation of the Antioxidant Activity, Deodorizing Effect, and Antibacterial Activity of ‘Porotan’ Chestnut By-Products and Establishment of a Compound Paper
Abstract
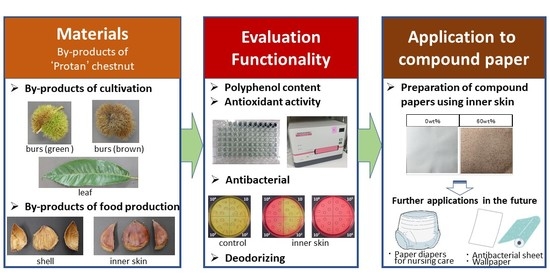
:1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. Japanese Chestnut ‘Porotan’
2.1.2. Effects of Chestnut Samples on Bacteria
2.1.3. Preparation of Compound Papers
2.2. Analysis of Properties
2.2.1. Extraction Method for Total Polyphenol Content (TPC), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), and Hydrophilic Oxygen Radical Absorbance Capacity (H-ORAC) Assays
2.2.2. TPC
2.2.3. DPPH Radical Scavenging Activity Assay
2.2.4. Hydrophilic-ORAC (H-ORAC)
2.2.5. Antibacterial Properties
2.2.6. Evaluation of Deodorant Activity
2.2.7. Scanning Electron Microscopy
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. TPC and Antioxidant Activity
3.2. Antibacterial Activity
3.3. Deodorizing Activity of the Inner Chestnut Skin and Compound Paper
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/?fbclid=IwAR0HODyYs-D3AYvKVIjfi3cUdlDYfkQjpT3tjtN5IE9KukB38Im_TUUxZMY#data/QC/visualize (accessed on 1 May 2021).
- Tsurunaga, Y.; Saito, M.; Yasugi, K.; Takahashi, T. Technical Development of Value-Added Processed Products Using Frozen-stored’Porotan, a New Variety of Chestnut. J. Jpn. Soc. Food Sci. 2018, 65, 111–117. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crop. Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Hwang, I.-K.; Park, J.-B. Analysis of physicochemical factors related to the automatic pellicle removal in Korean chestnut (Castanea crenata). J. Agric. Food Chem. 2001, 49, 6045–6049. [Google Scholar] [CrossRef]
- An, J.-Y.; Wang, L.-T.; Lv, M.-J.; Wang, J.-D.; Cai, Z.-H.; Wang, Y.-Q.; Zhang, S.; Yang, Q.; Fu, Y.-J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Youn, U.-Y.; Shon, M.-S.; Kim, G.-N.; Katagiri, R.; Harata, K.; Ishida, Y.; Lee, S.-C. Antioxidant and anti-adipogenic activities of chestnut (Castanea crenata) byproducts. Food Sci. Biotechnol. 2016, 25, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Shiromizu, T.; Katsui, M. Suppressive effect of polyphenols extracted from chestnut skins on lipid absorption in rats. Nippon Shokuhin Kagaku Kogaku Kaishi = J. Jpn. Soc. Food Sci. Technol. 2009, 56, 545–548. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. ISSN 2012, 2277, 2502. [Google Scholar]
- Gupta, P.; Mohammad, T.; Khan, P.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Hassan, M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019, 118, 109245. [Google Scholar] [CrossRef]
- Lee, N.-K.; Jung, B.S.; Yu, H.H.; Kim, J.-S.; Paik, H.-D. The impact of antimicrobial effect of chestnut inner shell extracts against Campylobacter jejuni in chicken meat. LWT-Food Sci. Technol. 2016, 65, 746–750. [Google Scholar] [CrossRef]
- Misuri, F.; Marri, L. Antibacterial activity of wood distillate from residual virgin chestnut biomass. Eur. J. Wood Wood Prod. 2021, 79, 237–239. [Google Scholar] [CrossRef]
- Ham, J.-S.; Kim, H.-Y.; Lim, S.-T. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind. Crop. Prod. 2015, 73, 99–105. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Nishio, S.; Yamada, M.; Sawamura, Y.; Sato, A.; Hirabayashi, T.; Saito, T. Inheritance of the easy-peeling pellicle trait of Japanese chestnut cultivar Porotan. HortScience 2012, 47, 845–847. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Kotobuki, K.; Sawamura, Y.; Abe, K.; Terai, O.; Shoda, M.; Takada, N.; Sato, Y.; Hirabayashi, T.; Sato, A. New Japanese Chestnut Cultivar ‘Porotan’; Bulletin of the National Institute of Fruit Tree Science: Tsukuba, Japan, 2009; pp. 1–9. [Google Scholar]
- Takahashi, T.; Aso, Y.; Kasai, W.; Kondo, T. Effect of light irradiation on the antibacterial activity of compounded papers containing wasted tea leaves. J. Wood Sci. 2010, 56, 299–306. [Google Scholar] [CrossRef]
- Takahashi, T.; Aso, Y.; Kasai, W.; Kondo, T. Improving the antibacterial activity against Staphylococcus aureus of composite sheets containing wasted tea leaves by roasting. J. Wood Sci. 2010, 56, 403–410. [Google Scholar] [CrossRef]
- Takahashi, T.; Aso, Y.; Kasai, W.; Kondo, T. Synergetic deodorant effect and antibacterial activity of composite paper containing waste tea leaves. J. Wood Sci. 2011, 57, 308–316. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsurunaga, Y.; Schmidt, W.F.; Yoshino, K. Functional evaluation of horse chestnut seed and its application in the production of compounded paper for effective utilization of an untapped resource. J. Wood Sci. 2017, 63, 484–495. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009, 113, 964–969. [Google Scholar] [CrossRef]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yamasaki, K.; Takano-Ishikawa, Y.; Hino, A.; Yasui, A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012, 28, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Aso, Y.; Yoshino, K. Incorporation of photocatalytic function into nonwoven polyester fabrics via impregnation with peroxo titanic acid solution. J. Mater. Sci. 2013, 48, 8199–8208. [Google Scholar] [CrossRef]
- Takahashi, T.; Oowaki, M.; Onohara, Y.; Yanagi, Y. Deodorant performance of titanium dioxide-added acrylic/cellulose diacetate blended fibers. Text. Res. J. 2013, 83, 800–812. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, L.H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Gordon, M.H.; Garcia-Macias, P.; Labrinea, H. Extraction and determination of ellagic acid contentin chestnut bark and fruit. Food Chem. 2008, 110, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Qu, H.; Li, L.; Mao, B.; Xu, Y.; Lin, X.; Luo, Z. Delaying the biosynthesis of aromatic secondary metabolites in postharvest strawberry fruit exposed to elevated CO2 atmosphere. Food Chem. 2020, 306, 125611. [Google Scholar] [CrossRef]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.G.; Sorrenti, V. Effects of ellagic acid on angiogenic factors in prostate cancer cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [Green Version]
- Nankar, R.P.; Doble, M. Hybrid drug combination: Anti-diabetic treatment of type 2 diabetic Wistar rats with combination of ellagic acid and pioglitazone. Phytomedicine 2017, 37, 4–9. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Liu, Y.; Liu, H.; Shang, Y. Evaluating effects of ellagic acid on the quality of kumquat fruits during storage. Sci. Hortic. 2018, 227, 244–254. [Google Scholar] [CrossRef]
- Noormandi, A.; Dabaghzadeh, F. Effects of green tea on Escherichia coli as a uropathogen. J. Tradit. Complement. Med. 2015, 5, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-L.; Cesario, T.; Wang, Y.; Shanbrom, E.; Thrupp, L. Antibacterial activity of vegetables and juices. Nutrition 2003, 19, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Cho, Y.; Schiller, N.; Kahng, H.; Oh, K. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- JIS-L-1902. Determination of Antibacterial Activity and Efficacy on Textile Products; Japanese Standard Association: Tokyo, Japan, 2015; pp. 1–37. (In Japanese) [Google Scholar]
- Eshtiaghi, M.N.; Kuldiloke, J.; Yoswathana, N. Deodorization of coconut oil using activated charcoal and charcoal regeneration. J. Food Agric. Environ. 2012, 10, 178–181. [Google Scholar]





| Species of Bacterium | Sample | Incubation Time (h) | Antibacterial Properties | Bacteria Yielded Growth Values *3 | |
|---|---|---|---|---|---|
| Viable Bacteria (CFU *1/mL) | Antibacterial Activity Value *2 | ||||
| Staphylococcus aureus | Before incubation | 0 | 1.00 × 105 | − | |
| Burs (green) | 18 | 3.84 × 106 | 3.43 | ||
| Burs (brown) | 18 | 5.80 × 106 | 3.25 | ||
| Shell | 18 | 1.36 × 106 | 3.21 | ||
| Inner skin | 18 | 2.04 × 105 | 4.03 | ||
| Leaf | 18 | 1.16 × 106 | 3.28 | ||
| Green tea | 18 | 1.80 × 109 | 0.76 | ||
| Only bacteria | 18 | 1.04 × 1010 | − | 5.02 | |
| Escherichia coli | Before incubation | 0 | 1.00 × 105 | − | |
| Burs (green) | 18 | 4.40 × 108 | 0.80 | ||
| Burs (brown) | 18 | 2.28 × 107 | 2.09 | ||
| Shell | 18 | 2.28 × 1010 | −0.91 | ||
| Inner skin | 18 | 2.16 × 108 | 1.11 | ||
| Leaf | 18 | 1.24 × 109 | 0.35 | ||
| Green tea | 18 | 4.64 × 108 | 0.78 | ||
| Only bacteria | 18 | 2.80 × 109 | − | 4.45 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurunaga, Y.; Takahashi, T. Evaluation of the Antioxidant Activity, Deodorizing Effect, and Antibacterial Activity of ‘Porotan’ Chestnut By-Products and Establishment of a Compound Paper. Foods 2021, 10, 1141. https://doi.org/10.3390/foods10051141
Tsurunaga Y, Takahashi T. Evaluation of the Antioxidant Activity, Deodorizing Effect, and Antibacterial Activity of ‘Porotan’ Chestnut By-Products and Establishment of a Compound Paper. Foods. 2021; 10(5):1141. https://doi.org/10.3390/foods10051141
Chicago/Turabian StyleTsurunaga, Yoko, and Tetsuya Takahashi. 2021. "Evaluation of the Antioxidant Activity, Deodorizing Effect, and Antibacterial Activity of ‘Porotan’ Chestnut By-Products and Establishment of a Compound Paper" Foods 10, no. 5: 1141. https://doi.org/10.3390/foods10051141
APA StyleTsurunaga, Y., & Takahashi, T. (2021). Evaluation of the Antioxidant Activity, Deodorizing Effect, and Antibacterial Activity of ‘Porotan’ Chestnut By-Products and Establishment of a Compound Paper. Foods, 10(5), 1141. https://doi.org/10.3390/foods10051141