Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Raw Material
2.3. Raw Material Chemical Composition
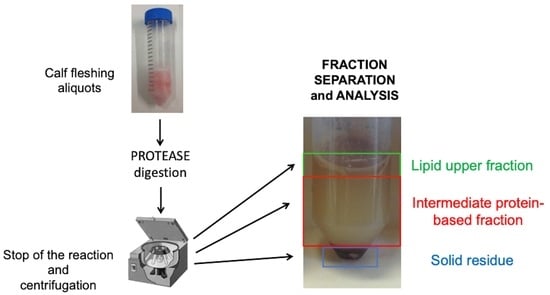
2.4. Proteases Digestion
2.5. Quantification of Free and Total Amino Acids, SDS-PAGE and Degree of Hydrolysis (DH) Analyses
2.6. Peptide Identification by Linear Trap Quadrupole (LTQ)-Orbitrap Analyses
2.7. Fatty Acid Analysis
2.8. Antioxidant and Anti-Tyrosinase Activity Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Calf Fleshing Bulk Characterization
3.2. Optimization of the Enzymatic Reaction
3.3. Protein Fraction Analysis
3.4. Antioxidant and Anti-Tyrosinase Activity
3.5. Fatty Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Alternative Pathways to 2050. Available online: http://tools.foodsecurityportal.org/fao-global-perspectives-studies 2019 (accessed on 20 January 2021).
- FAO. SAVE FOOD: Global Initiative on Food Loss and Waste Reduction. 2021. Available online: http://www.fao.org/save-food/resources/en/ (accessed on 20 January 2021).
- Ockerman, H.W.; Hansen, C.L. Introduction and history of processing animal by-products. In Animal By-Products Processing and Utilization; Ockerman, H.W., Hansen, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–22. [Google Scholar]
- Russ, W.; Meyer-Pittroff, R. Utilizing waste products from the food production and processing industries. Crit. Rev. Food Sci. Nutr. 2004, 44, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by-products and waste materials from meat, poultry and fish processing industries: a review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Alvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Bajza, Z.; Vrucek, V. Thermal and enzymatic recovering of proteins from untanned leather waste. Waste Manag. 2001, 21, 79–84. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Olsen, K.; Grossi, A.; Otte, J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013, 141, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Damrongsakkul, S.; Ratanathammapan, K.; Komolpis, K.; Tanthapanichakoon, W. Enzymatic hydrolysis of rawhide using papain and neutrase. J. Ind. Eng. Chem. 2008, 14, 202–206. [Google Scholar] [CrossRef]
- Jian, S.; Wenyi, T.; Wuyong, C. Ultrasound-accelerated enzymatic hydrolysis of solid leather waste. J. Clean Prod. 2008, 16, 591–597. [Google Scholar] [CrossRef]
- Anzani, C.; Prandi, B.; Tedeschi, T.; Baldinelli, C.; Sorlini, G.; Wierenga, P.A.; Dossena, A.; Sforza, S. Degradation of collagen increases nitrogen solubilisation during enzymatic hydrolysis of fleshing meat. Waste Biomass. Valor. 2018, 9, 1113–1119. [Google Scholar] [CrossRef]
- Toldra, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Arihara, K.; Ohata, M. Functional meat products. In Handbook of Meat Processing; Toldrá, F., Ed.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 423–439. [Google Scholar]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Wang, B.-S.; Chang, L.-W.; Wu, H.-C.; Huang, S.-L.; Chu, H.-L.; Huang, M.-H. Antioxidant and anti-tyrosinase activity of aqueous extracts of green asparagus. Food Chem. 2011, 127, 141–146. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, W. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Rxob.) Blume extract. J. Food Sci. Technol. 2013, 78, C1354–1362. [Google Scholar]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitrophotoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Protopappa, A.; Megoulas, N.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorgan. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, M.; Graen-Heedfeld, J.; Bretz, K.; Guillon, F.; Michelini, E.; Calabretta, M.M.; Lamborghini, M.; Gruarin, N.; Roda, A.; Kraft, A.; et al. Peptide fractions obtained from rice by-products by means of an environment-friendly process show in vitro health-related bioactivities. PLoS ONE 2017, 12, e0170954. [Google Scholar] [CrossRef] [Green Version]
- Schurink, M.; van Berkel, W.; Wichers, H.; Boeriu, C. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef]
- Mapiye, C.; Dugan, M.E.R.; Juarez, M.; Basarab, J.A.; Baron, V.S.; Turner, T.; Yang, X.; Aldai, N.; Aalhus, J.L. Influence of alpha-tocopherol supplementation on trans-18:1 and conjugated linoleic acid profiles in beef from steers fed a barley-based diet. Animal 2012, 6, 1888–1896. [Google Scholar] [CrossRef] [Green Version]
- Marzocchi, S.; Pasini, F.; Baldinelli, C.; Caboni, M.F. Value-addition of beef meat by-products: Lipid characterization by chromatographic techniques. J. Oleo Sci. 2018, 67, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. Official Methods of Analysis. 2002. Available online: http://www.aoac.org/ (accessed on 14 December 2020).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Verardo, V.; Gomez-Caravaca, A.M.; Gori, A.; Losi, G.; Caboni, M.F. Bioactive lipids in the butter production chain from Parmigiano Reggiano cheese area. J. Sci. Food Agr. 2013, 93, 3625–3633. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Rai, A.K.; Nived, C.; Sakhare, P.Z.; Suresh, P.V.; Bhaskar, N.; Mahendrakar, N.S. Optimization of acid hydrolysis conditions of delimed tannery fleshings by response surface method. J. Sci. Ind. Res. India 2009, 68, 967–974. [Google Scholar]
- Bhaskar, N.; Sakhare, P.Z.; Suresh, P.V.; Gowda, L.R.; Mahendrakar, N.S. Biostabilization and preparation of protein hydrolysates from delimed leather fleshings. J. Sci. Ind. Res. India 2007, 66, 1054–1063. [Google Scholar]
- USDA-United States Department of Agriculture. Agricultural Research Service, National Nutrient Database for Standard Reference Legacy. Available online: https://fdc.nal.usda.gov (accessed on 14 December 2020).
- White, J.S.; White, D.C. Source Book of Enzymes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Olsen, J.V.; Ong, S.E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Haslaniza, H.; Maskat, M.Y.; Aida, W.M.W.; Mamot, S.; Saadiah, I. Optimization of enzymatic hydrolysis of cockle (Anadara Granosa) meat wash water precipitate for the development of seafood flavor. Int. Food Res. J. 2013, 20, 3053–3059. [Google Scholar]
- Ryder, K.; Bekhit, A.E.D.; McConnell, M.; Carne, A. Towards generation of bioactive peptides from meat industry waste proteins: Generation of peptides using commercial microbial proteases. Food Chem. 2016, 208, 42–50. [Google Scholar] [CrossRef]
- Choi, J.S.; Moon, H.E.; Roh, M.K.; Ha, V.M.; Lee, B.B.; Cho, K.K.; Choi, I.S. Physiological properties of Scomber japonicus meat hydrolysate prepared by subcritical water hydrolysis. J. Environ. Biol. 2016, 37, 57–63. [Google Scholar]
- Baggio, S.R.; Bragagnolo, N. The effect of heat treatment on the cholesterol oxides, cholesterol, total lipid and fatty acid contents of processed meat products. Food Chem. 2006, 95, 611–619. [Google Scholar] [CrossRef]
- Muchenje, V.; Hugo, A.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Raats, J.G. Cholesterol levels and fatty acid profiles of beef from three cattle breeds raised on natural pasture. J. Food Compos. Anal. 2009, 22, 354–358. [Google Scholar] [CrossRef]
- Brugiapaglia, A.; Lussiana, C.; Destefanis, G. Fatty acid profile and cholesterol content of beef at retail of Piemontese, Limousin and Friesian breeds. Meat Sci. 2014, 96, 568–573. [Google Scholar] [CrossRef] [PubMed]


| Amino Acids | % on Total Amino Acids | Reference (%) |
|---|---|---|
| Hyp | 12.1 ± 0.6 | 1.4 |
| Asp | 7.0 ± 0.5 | 9.4 |
| Ser | 3.2 ± 0.1 | 4.2 |
| Glu | 13.2 ± 0.7 | 15.6 |
| Gly | 8.2 ± 0.6 | 7.1 |
| His | 2.2 ± 0.3 | 3.4 |
| Arg | 6.3 ± 0.5 | 6.8 |
| Thr | 3.4 ± 0.3 | 4.0 |
| Ala | 5.3 ± 0.8 | 6.5 |
| Pro | 10.7 ± 1.2 | 5.3 |
| Tyr | 2.7 ± 0.4 | 3.2 |
| Val | 3.3 ± 0.2 | 5.1 |
| Met | 3.4 ± 0.3 | 2.7 |
| Lys | 6.6 ± 0.4 | 8.6 |
| Ile | 3.3 ± 0.3 | 4.6 |
| Leu | 5.8 ± 0.5 | 8.1 |
| Phe | 3.4 ± 0.3 | 4.1 |
| Amino Acids | Papain | Pancreatin | Bromelain | Trypsin (B) |
|---|---|---|---|---|
| Hyp | 3.46 ± 0.33 b | 3.54 ± 0.07 b | 2.79 ± 0.60 b | 5.75 ± 0.30 a |
| Asp | 0.14 ± 0.03 c | 1.81 ± 0.01 a | 0.81 ± 0.01 b | 1.78 ± 0.36 a |
| Ser | 3.55 ± 0.06 a | 4.17 ± 0.66 a | 3.73 ± 0.20 a | 1.75 ± 0.01 b |
| Glu | 4.66 ± 0.04 b | 6.86 ± 0.25 a | 2.44 ± 0.37 c | 2.77 ± 0.05 c |
| Gly | 9.00 ± 0.02 a | 1.67 ± 0.15 c | 3.27 ± 0.13 b | 1.57 ± 0.04 c |
| His | 2.62 ± 0.06 c | 5.80 ± 0.08 a | 4.65 ± 0.16 b | 2.47 ± 0.02 c |
| Arg | 14.58 ± 0.29 a | 16.63 ± 1.69 a | 3.74 ± 0.34 b | 14.27 ± 1.04 a |
| Thr | 2.93 ± 0.08 b | 3.79 ± 0.07 b | 8.37 ± 0.24 a | 3.48 ± 0.05 b |
| Ala | 3.44 ± 0.03 b | 3.70 ± 0.67 a | 2.29 ± 0.09 b | 3.28 ± 0.80 b |
| Pro | 7.67 ± 0.04 b | 1.32 ± 0.23 c | 11.89 ± 0.62 a | 1.36 ± 0.21 c |
| Tyr | 7.89 ± 0.21 a | 8.93 ± 0.86 a | 4.96 ± 0.47 b | 7.52 ± 0.73 a |
| Val | 1.85 ± 0.09 c | 4.43 ± 0.15 a | 3.08 ± 0.01 b | 3.46 ± 0.69 b |
| Met | 3.93 ± 0.13 b | 4.20 ± 0.41 b | 4.55 ± 0.22 b | 5.69 ± 0.03 a |
| Lys | 15.36 ± 0.13 b | 14.89 ± 1.84 b | 10.55 ± 0.06 c | 22.51 ± 1.89 a |
| Ile | 3.96 ± 0.12 b | 4.05 ± 0.20 b | 19.26 ± 0.23 a | 4.47 ± 0.69 b |
| Leu | 11.20 ± 0.47 a | 7.82 ± 0.33 c | 9.04 ± 0.16 b | 9.45 ± 0.38 b |
| Phe | 3.75 ± 0.33 c | 6.40 ± 0.58 b | 4.59 ± 0.09 c | 8.40 ± 0.45 a |
| Enzyme | Identified Peptides | Parent Protein | Average Peptide Length (N°AA) |
|---|---|---|---|
| Papain | 78 | Collagen alpha-1(I) chain | 14 |
| 71 | Actin, alpha skeletal muscle | 11 | |
| 56 | Collagen alpha-2(I) chain | 13 | |
| 54 | Collagen alpha-1(II) chain | 12 | |
| 30 | Myosin-1 | 11 | |
| Pancreatin | 70 | Titin | 12 |
| 57 | Collagen alpha-1(I) chain | 11 | |
| 53 | Collagen alpha-2(I) chain | 10 | |
| 29 | Collagen, type III, alpha 1 | 14 | |
| 24 | IgG heavy chain | 18 | |
| Bromelain | 24 | Collagen alpha-1(I) | 9 |
| 17 | Hemoglobin subunit beta | 23 | |
| 15 | Hemoglobin subunit alpha | 25 | |
| 15 | Collagen alpha-2(I) chain | 9 | |
| 13 | Myosin light chain 1/3, skeletal muscle isoform | 13 | |
| Trypsin (B) | 59 | Titin | 11 |
| 46 | Myosin-2 | 11 | |
| 28 | Myosin-1 | 10 | |
| 23 | Collagen alpha-1(I) chain | 13 | |
| 10 | Myosin-7 | 12 |
| FA | Papain | Pancreatin | Bromelain | Trypsin (B) |
|---|---|---|---|---|
| C12:0 | 0.69 ± 0.00 | n.d. | n.d | 0.51 ± 0.02 |
| C14:0 | 5.82 ± 0.09 | 5.88 ± 0.04 | 5.66 ± 0.30 | 5.41 ± 0.33 |
| C14:1c | 2.30 ± 0.19 | 2.67 ± 0.56 | 3.79 ± 0.15 | 2.14 ± 0.01 |
| C15:0 | 0.31 ± 0.01 | n.d | n.d | 0.28 ± 0.00 |
| C16:0 | 24.66 ± 0.03 | 23.99 ± 1.95 | 26.55 ± 0.38 | 24.72 ± 0.19 |
| C16:1 t | 0.37 ± 0.02 | n.d | n.d | 0.27 ± 0.07 |
| C16:1 c | 5.80 ± 0.02 | 5.89 ± 0.60 | 5.88 ± 0.96 | 5.92 ± 0.28 |
| C17:0 | 0.58 ± 0.01 | n.d | n.d | 0.59 ± 0.07 |
| C17:1 | 0,61 ± 0.04 | n.d | n.d | 0.64 ± 0.10 |
| C18:0 | 7.77 ± 0.03 | 9.91 ± 0.66 | 8.21 ± 0.47 | 7.59 ± 0.40 |
| C18:1 t9 | 1.56 ± 0.11 | n.d | n.d | n.d |
| C18:1 c9 | 40.52 ± 0.02 | 39.59 ± 4.69 | 42.80 ± 1.97 | 41.61 ± 1.37 |
| C18:1 c11 | 1.99 ± 0.14 | 2.06 ± 0.42 | 2.12 ± 0.32 | 2.13 ± 0.10 |
| C18:2 n6 | 4.91 ± 0.15 | 4.78 ± 0.38 | 4.99 ± 0.95 | 4.38 ± 0.60 |
| C18:3 n3 | 0.71 ± 0.08 | n.d | n.d | 0.90 ± 0.08 |
| C20:0 | 0.64 ± 0.06 a | n.d | n.d | 0.42 ± 0.01 b |
| C20:3 n6 | 0.76 ± 0.66 | 5.24 ± 0.77 | n.d | 0.85 ± 0.10 |
| SFA | 40.46 ± 0.08 | 39.77 ± 0.75 | 40.41 ± 1.16 | 39.52 ± 0.98 |
| MUFA | 53.16 ± 0.51 | 50.20 ± 3.15 | 54.60 ± 2.11 | 54.36 ± 1.40 |
| PUFA | 6.39 ± 0.59 b | 10.03 ± 2.39 a | 4.99 ± 0.95 c | 6.12 ± 0.42 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, T.; Anzani, C.; Ferri, M.; Marzocchi, S.; Caboni, M.F.; Monari, S.; Tassoni, A. Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods 2021, 10, 755. https://doi.org/10.3390/foods10040755
Tedeschi T, Anzani C, Ferri M, Marzocchi S, Caboni MF, Monari S, Tassoni A. Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods. 2021; 10(4):755. https://doi.org/10.3390/foods10040755
Chicago/Turabian StyleTedeschi, Tullia, Cecilia Anzani, Maura Ferri, Silvia Marzocchi, Maria Fiorenza Caboni, Stefania Monari, and Annalisa Tassoni. 2021. "Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids" Foods 10, no. 4: 755. https://doi.org/10.3390/foods10040755
APA StyleTedeschi, T., Anzani, C., Ferri, M., Marzocchi, S., Caboni, M. F., Monari, S., & Tassoni, A. (2021). Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods, 10(4), 755. https://doi.org/10.3390/foods10040755