Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stability of Naringenin and Naringin in Alkaline Condition
2.3. Effect of pH Shift on the Solubility of Naringenin and Naringin
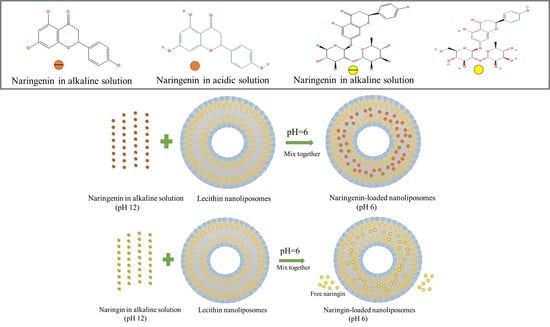
2.4. Narigenin-Loaded and Naringin-Loaded Nanoliposome Preparation
2.5. Encapsulation Efficiency and Loading Capacity of Naringenin and Naringin
2.6. Naringenin-Loaded and Naringin-Loaded Nanoliposomes Characterization
2.7. Storage Stability
2.8. Atomic Force Microscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Stability of Naringenin and Naringin in Alkaline Conditions
3.2. Solubility of Naringenin and Naringin with pH-Shift
3.3. Encapsulation Efficiency (EE) and Loading Capacity (LC) of Naringenin and Naringin in Nanoliposomes
3.4. Characterization of Naringenin-Loaded and Naringin-Loaded Nanoliposomes
3.5. Stability of Naringenin-Loaded Nanoliposomes
3.6. Micosturcture of Naringenin-Loaded Nanoliposomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raeisib, S.; Chavoshia, H.; Mohammadic, M.; Ghorbanid, M.; Sabzichie, M.; Ramezani, F. Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem. 2019, 83, 168–175. [Google Scholar] [CrossRef]
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Shafiullah; Ahmed, S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for naringin loading: A morphological investigation through afm. Carbohyd. Polym. 2017, 174, 243. [Google Scholar] [CrossRef] [PubMed]
- Hermenean, A.; Ardelean, A.; Stan, M.; Herman, H.; Mihali, C.V.; Costache, M.; Dinischiotu, A. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem. Biol. Interact. 2013, 205, 138–147. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.D.; Epifano, F.; Grenier, D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal Res. 2008, 43, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Jresat, I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology 2016, 97, 224–232. [Google Scholar] [CrossRef]
- Yen, H.R.; Liu, C.J.; Yeh, C.C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S.; Wang, N.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R.; et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010, 235, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Ni, C.C.; Yin, M.C.; Lii, C.K. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine 2012, 59, 65–71. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [Green Version]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Pérez-Abril, M.; Castillo, J.; Serrano, A.; Mercader, M.T.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of temperature, pH, β- and HP-β-cds on the solubility and stability of flavanones: Naringenin and hesperetin. LWT Food Sci.Technol. 2019, 108, 233–239. [Google Scholar] [CrossRef]
- Uchiyama, H.; Kadota, K.; Nakanishi, A.; Tandia, M.; Tozuka, Y. A simple blending with α-glycosylated naringin produces enhanced solubility and absorption of pranlukast hemihydrate. Int. J. Pharmaceut. 2019, 567, 118490. [Google Scholar] [CrossRef]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin–naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Budel, R.G.; Silva, D.A.D.; Moreira, M.P.; Dalcin, A.J.F.; Boeck, C.R. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf. B Biointerfaces 2020, 188, 110754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Wang, S.C.; Firempong, C.K.; Zhang, H.Y.; Wang, M.M.; Zhang, Y.; Zhu, Y.; Yu, J.N.; Xu, X.M. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Fu, X.; Cheng, H.; Wusigale; Liang, L. α-Tocopherol and naringenin in whey protein isolate particles: Partition, antioxidant activity, stability and bioaccessibility. Food Hydrocoll. 2020, 106, 105895. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter. 2014, 10, 6820–6830. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bayram, I.; Zhang, W.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Gao, Y. Fabrication and characterization of curcumin-loaded pea protein isolate-surfactant complexes at neutral pH. Food Hydrocoll. 2021, 111, 106214. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; Mcclements, D.J. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Formation, physicochemical stability, and redispersibility of curcumin-loaded rhamnolipid nanoparticles using the pH-driven method. J. Agric. Food Chem. 2020, 68, 7103–7111. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; Mcclements, D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Zou, L.; Liu, C.; Mcclements, D.J. Fabrication and characterization of curcumin-loaded liposomes formed from sunflower lecithin: Impact of composition and environmental stress. J. Agric. Food Chem. 2018, 66, 12421–12430. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Zhou, W.; Liu, W.; Mcclements, D.J. Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. J. Agric. Food Chem. 2019, 67, 7506–7511. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; He, Y.; Gu, J. Analytical methods for investigating in vivo fate of nanoliposomes: A review. J. Pharm. Anal. 2018, 8, 219–225. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Biol. 2010, 60, 29–50. [Google Scholar]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Xia, G.; Xue, F.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7, 8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2019, 146, 179–192. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Li, Z.; Hu, X.; Chen, X.; Liu, C. Hybrid liposomes composed of amphiphilic chitosan and phospholipid: Preparation, stability and bioavailability as a carrier for curcumin. Carbohydr. Polym. 2017, 156, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, C.; Zhai, J.; Yang, Y.; Yu, H. Ascorbyl palmitate effects on the stability of curcumin-loaded soybean phosphatidylcholine liposomes. Food Biosci. 2021, 41, 100923. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102237. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a nextgeneration drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homayoonfal, M.; Mousavi, S.M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Encapsulation of berberis vulgaris anthocyanins into nanoliposome composed of rapeseed lecithin: A comprehensive study on physicochemical characteristics and biocompatibility. Foods 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dai, T.; Tan, Y.; Fu, G.; Wan, Y.; Liu, C.; Mcclements, D.J. Fabrication of pea protein-tannic acid complexes: Impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 2020, 310, 125828. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dai, T.; Zhou, W.; Fu, G.; Wan, Y.; McClements, D.J.; Li, J. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res. Int. 2020, 136, 109618. [Google Scholar] [CrossRef]









| Naringin Concentration (mg/mL) | Mean Particle Size (nm) | PDI | ζ-Potentials (mV) |
|---|---|---|---|
| Blank nanoliposomes | 34.93 ± 0.98 a | 0.262 ± 0.012 b | −16.74 ± 1.87 a |
| 1.0 (1% w/v lecithin) | 34.03 ± 0.69 a | 0.230 ± 0.010 a | −12.94 ± 1.31 a |
| 1.5 (1% w/v lecithin) | 34.25 ± 0.72 a | 0.237 ± 0.010 a,b | −16.16 ± 1.60 a |
| 2.0 (1% w/v lecithin) | 35.09 ± 0.86 a | 0.240 ± 0.012 a,b | −13.90 ± 1.07 a |
| 3.0 (2% w/v lecithin) | 35.71 ± 0.49 a | 0.260 ± 0.011 b | −16.97 ± 1.48 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Li, R.; Gao, Y.; Zheng, Y.; Liao, L.; Cao, Y.; Li, J.; Zhou, W. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods 2021, 10, 963. https://doi.org/10.3390/foods10050963
Chen M, Li R, Gao Y, Zheng Y, Liao L, Cao Y, Li J, Zhou W. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods. 2021; 10(5):963. https://doi.org/10.3390/foods10050963
Chicago/Turabian StyleChen, Mianhong, Ruyi Li, Yuanyuan Gao, Yeyu Zheng, Liangkun Liao, Yupo Cao, Jihua Li, and Wei Zhou. 2021. "Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds" Foods 10, no. 5: 963. https://doi.org/10.3390/foods10050963
APA StyleChen, M., Li, R., Gao, Y., Zheng, Y., Liao, L., Cao, Y., Li, J., & Zhou, W. (2021). Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods, 10(5), 963. https://doi.org/10.3390/foods10050963