Rice Bran Fermentation Using Lactiplantibacillus plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food
Abstract
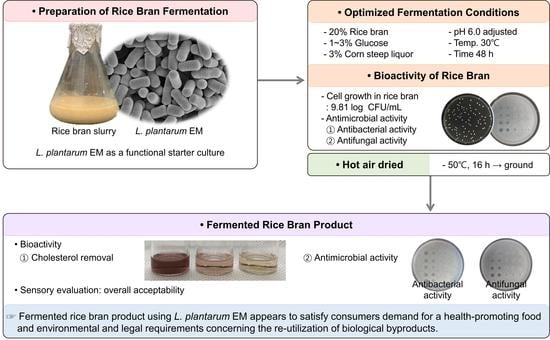
:1. Introduction
2. Materials and Methods
2.1. Microbial Cultures and Media
2.2. Optimization of Rice Bran Fermentation Conditions
2.2.1. Rice Bran Concentration in Rice Bran Slurry
2.2.2. Rice Bran Slurry Supplementation
2.2.3. pH and Temperature
2.2.4. Viable Cells and pH
2.2.5. Antimicrobial Activities
2.3. Preparation of Fermented Rice Bran
2.4. Cholesterol Removal
2.5. Phytase Activity and Phytic Acid
2.6. Sensory Evaluations
2.7. Statistical Analysis
3. Results and Discussion
3.1. Rice Bran Fermentation
3.1.1. Rice Bran as a Nutrient Source for LAB Cell Growth
3.1.2. Antimicrobial Activity of Fermented Rice Brans
3.1.3. Effects of pH and Temperature
3.2. Characterization of Rice Bran Products
3.2.1. Cholesterol Removal by Fermented Rice Bran Products
3.2.2. Antimicrobial Activity
3.2.3. Phytic Acid
3.2.4. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirci, T.; Aktaş, K.; Sözeri, D.; Öztürk, H.İ.; Akın, N. Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. J. Funct. Foods 2017, 36, 396–403. [Google Scholar] [CrossRef]
- Yang, M.; Ashraf, J.; Tong, L.; Wang, L.; Zhang, L.; Li, N.; Zhou, S.; Liu, L. Effects of Rhizopus oryzae and Aspergillus oryzae on prebiotic potentials of rice bran pretreated with superheated steam in an in vitro fermentation system. LWT 2021, 139, 110482. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Denardi de Souza, T.; Collazzo, C.C.; Badiale Furlong, E.; Souza Soares, L.A. The impact of Rhizopus oryzae cultivation on rice bran: Gamma-oryzanol recovery and its antioxidant properties. Food Chem. 2017, 228, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ritthibut, N.; Oh, S.J.; Lim, S.T. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT 2021, 135, 110273. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Dong, L.; Jia, X.; Liu, L.; Huang, F.; Chi, J.; Xiao, J.; Zhang, M.; Zhang, R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT Food Sci. Technol. 2019, 115, 108461. [Google Scholar] [CrossRef]
- Jeon, Y.B.; Lee, J.J.; Chang, H.C. Characterization of juice fermented with Lactobacillus plantarum EM and its cholesterol-lowering effects on rats fed a high-fat and high-cholesterol diet. Food Sci. Nutr. 2019, 7, 3622–3634. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.; Liu, C.; Yin, T.; Yan, X.; Wu, C.; He, H.; Yi, C. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef]
- Hasani, S.; Khodadadi, I.; Heshmati, A. Viability of Lactobacillus acidophilus in rice bran-enriched stirred yoghurt and the physicochemical and sensory characteristics of product during refrigerated storage. Int. Food Sci. Technol. 2016, 51, 2485–2492. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Deng, Y.; Zhang, Y.; Xiao, J.; Huang, F.; Wen, W.; Zhang, M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with α-amylase. Food Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef]
- Yun, J.S.; Wee, Y.J.; Kim, J.N.; Ryu, H.W. Fermentative production of DL-lactic acid from amylase-treated rice and wheat brans hydrolysate by a novel lactic acid bacterium, Lactobacillus sp. Biotechnol. Lett. 2004, 26, 1613–1616. [Google Scholar] [CrossRef]
- Gao, M.T.; Kaneko, M.; Hirata, M.; Toorisaka, E.; Hano, T. Utilization of rice bran as nutrient source for fermentative lactic acid production. Bioresour. Technol. 2008, 99, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Moon, S.H.; Chang, H.C. Characterization of high-ornithine-producing Weissella koreensis DB1 isolated from kimchi and its application in rice bran fermentation as a starter culture. Foods 2020, 9, 1545. [Google Scholar]
- Kwon, S.; Lee, P.C.; Lee, E.G.; Chang, Y.K.; Chang, N. Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate. Enzym. Microb. Technol. 2000, 26, 209–215. [Google Scholar] [CrossRef]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Mun, S.Y.; Kim, S.K.; Woo, E.R.; Chang, H.C. Purification and characterization of an antimicrobial compound produced by Lactobacillus plantarum EM showing both antifungal and antibacterial activities. LWT Food Sci. Technol. 2019, 114, 108403. [Google Scholar] [CrossRef]
- Park, S.; Son, H.K.; Chang, H.C.; Lee, J.J. Effects of cabbage-apple juice fermented by Lactobacillus plantarum EM on liqid profile improvement and obesity amelioration in rats. Nutrients 2020, 12, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudel, L.L.; Morris, M.D. Determination of cholesterol using ο-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar] [CrossRef]
- Quan, C.; Zhang, L.; Wang, Y.; Ohta, Y. Production of phytase in a low phosphate medium by a novel yeast Candida krusei. J. Biosci. Bioeng. 2001, 92, 154–160. [Google Scholar] [CrossRef]
- Rivas, B.; Moldes, B.; Domínguez, J.M.; Parajó, J.C. Development of culture media containing spent yeast cells of Debaryomyces hansenii and corn steep liquor for lactic acid production with Lactobacillus rhamnosus. Int. J. Food. Microbiol. 2004, 97, 93–98. [Google Scholar] [CrossRef]
- Liggett, R.W.; Koffler, H. Corn steep liquor in microbiology. Bacteriol. Rev. 1948, 12, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.M.; Wisbeck, E.; Hoch, I.; Jonas, R. Production of glucose-fructose oxidoreductase and ethanol by Zymomonas mobilis ATCC 29191 in medium containing corn steep liquor as a source of vitamins. Appl. Microbiol. Biotechnol. 2001, 55, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Amartey, S.; Jeffries, T.W. Comparison of corn steep liquor with other nutrients in the fermentation of d-xylose by Pichia stipites CBS 6054. Biotechnol. Lett. 1994, 16, 211–214. [Google Scholar] [CrossRef]
- Lee, P.C.; Lee, W.G.; Lee, S.Y.; Chang, H.N.; Chang, Y.K. Fermentative production of succinic acid from glucose and corn steep liquor by Anaerobiospirillum succiniciproducens. Biotechnol. Bioprocess Eng. 2000, 5, 379–381. [Google Scholar] [CrossRef]
- Cheilas, T.; Stoupis, T.; Christakopoulos, P.; Katapodis, P.; Mamma, D.; Hatzinikolaou, D.G.; Kekos, D.; Macris, B.J. Hemi-cellulolytic activity of Fusarium oxysporum grown on sugar beet pulp. Process Biochem. 2000, 35, 557–561. [Google Scholar] [CrossRef]
- Demirci, A.; Pometto, A.L.; Lee, B.; Hinz, P.N. Media evaluation of lactic acid repeated-batch fermentation with Lactobacillus plantarum and Lactobacillus casei subsp. rhamnosus. J. Agric. Food Chem. 1998, 46, 4771–4774. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, Y.Y. Effect of temperature and various nitrogen sources on L (+)-lactic acid production by Lactobacillus casei. Appl. Microbiol. Biotechnol. 1996, 45, 307–313. [Google Scholar] [CrossRef]
- Arasaratnam, V.; Senthuran, A.; Balasubramaniam, K. Supplementation of whey with glucose and different nitrogen sources for lactic acid production by Lactobacillus delbrueckii. Enzym. Microb. Technol. 1996, 19, 482–486. [Google Scholar] [CrossRef]
- Nancib, N.; Nancib, A.; Boudjelal, A.; Benslimane, D.; Blanchard, F.; Boudrant, J. The effect of supplementation by different nitrogen sources on the production of lactic acid from data juice by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 2001, 78, 149–153. [Google Scholar] [CrossRef]
- Rohrer, C.A.; Siebenmorgen, T.J. Nutraceutical concentrations within the bran of various rice kernel thickness fractions. Biosyst. Eng. 2004, 88, 453–460. [Google Scholar] [CrossRef]
- Stiles, J.; Penkar, S.; Plocková, M.; Chumchalová, J.; Bullerman, L.B. Antifungal activity of sodium acetate and Lactobacillus rhamnosus. J. Food Prot. 2002, 65, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Nissar, J.; Ahad, T.; Naik, H.R.; Hussain, S.Z. A review phytic acid: As antinutrient or nutraceutical. J. Pharm. Phytochem. 2017, 6, 1554–1560. [Google Scholar]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
| Culture | Viable Cells (log CFU/mL) | |
|---|---|---|
| Nutrient | Concentration (%) | |
| MRS (control) | 9.59 ± 0.13 A | |
| Rice bran (RB) | 5 | 8.58 ± 0.13 a |
| 10 | 8.39 ± 0.41 a | |
| 15 | 8.64 ± 0.39 a | |
| 20 | 8.67 ± 0.38 aB | |
| 25 | 8.36 ± 0.01 a | |
| 30 | 8.30 ± 0.01 a | |
| 20% RB + Carbon-source | ||
| Glucose | 1 | 8.69 ± 0.16 aB |
| 3 | 8.66 ± 0.10 a | |
| Maltose | 1 | 8.62 ± 0.02 a |
| 3 | 8.63 ± 0.05 a | |
| Sucrose | 1 | 8.65 ± 0.12 a |
| 3 | 8.60 ± 0.04 a | |
| Fructose | 1 | 8.63 ± 0.05 a |
| 3 | 8.62 ± 0.12 a | |
| 20% RB + Nitrogen-source | ||
| Peptone | 1 | 8.86 ± 0.19 a |
| 3 | 8.94 ± 0.06 ab | |
| Beef extract | 1 | 8.79 ± 0.11 a |
| 3 | 9.05 ± 0.03 aB | |
| Yeast extract | 1 | 8.84 ± 0.12 a |
| 3 | 8.76 ± 0.04 a | |
| Soytone | 1 | 8.81 ± 0.04 a |
| 3 | 8.89 ± 0.04 ab | |
| 20% RB + Complex compound source | ||
| Corn steep liquor (CSL) | 1 | 9.29 ± 0.18 b |
| 2 | 9.33 ± 0.11 b | |
| 3 | 9.70 ± 0.11 aA | |
| 4 | 9.38 ± 0.09 b | |
| 5 | 9.40 ± 0.09 b | |
| 20% RB + Combined nutrients | ||
| 20% RB + 1% Glucose + 3% Beef extract | 9.03 ± 0.28 b | |
| 20% RB + 1% Glucose + 3% CSL | 9.78 ± 0.06 aA | |
| 20% RB +3% Beef extract + 3% CSL | 9.60 ± 0.05 aA | |
| 20% RB + 1% Glucose + 3% CSL + 3% Beef extract | 9.65 ± 0.12 aA | |
| Culture. | Antimicrobial Activity (AU/mL) | ||
|---|---|---|---|
| Nutrient | Concentration (%) | Antibacterial | Antifungal |
| MRS (control) | 300 | 600 | |
| Rice bran (RB) | 5 | 0 | 0 |
| 10 | 0 | 0 | |
| 15 | 0 | 0 | |
| 20 | 0 | 0 | |
| 25 | 0 | 0 | |
| 30 | 0 | 0 | |
| 20% RB + Carbon-source | |||
| Glucose | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Maltose | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Sucrose | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Fructose | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| 20% RB + Nitrogen-source | |||
| Peptone | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Beef extract | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Yeast extract | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| Soytone | 1 | 100 | 0 |
| 3 | 100 | 0 | |
| 20% RB + Complex compound source | |||
| Corn steep liquor (CSL) | 1 | 100 | 100 |
| 2 | 200 | 100 | |
| 3 | 200 | 100 | |
| 4 | 200 | 100 | |
| 5 | 200 | 100 | |
| 20% RB + Combined nutrients | |||
| 20% RB + 1% Glucose + 3% Beef extract | 100 | 0 | |
| 20% RB + 1% Glucose + 3% CSL | 200 | 400 | |
| 20% RB + 3% Beef extract + 3% CSL | 200 | 400 | |
| 20% RB + 1% Glucose + 3% CSL + 3% Beef extract | 200 | 400 | |
| Factor | Viable Cells (CFU/mL) | Antimicrobial Activity (AU/mL) | ||||
|---|---|---|---|---|---|---|
| Antibacterial | Antifungal | |||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Temp. (°C) * | ||||||
| 25 | 9.49 ± 0.08 b | 9.28 ± 0.25 a | 100 | 200 | 100 | 200 |
| 30 | 9.78 ± 0.11 a | 9.33 ± 0.21 a | 200 | 200 | 200 | 400 |
| 37 | 9.47 ± 0.06 b | 8.69 ± 0.09 b | 100 | 200 | 100 | 200 |
| pH ** | ||||||
| 4.0 | 9.39 ± 0.10 c | 8.90 ± 0.30 cd | 100 | 100 | 100 | 100 |
| 5.0 | 9.44 ± 0.17 c | 9.22 ± 0.15 bc | 100 | 200 | 100 | 100 |
| 6.0 | 9.81 ± 0.10 a | 9.59 ± 0.07 a | 200 | 200 | 200 | 400 |
| 7.0 | 9.76 ± 0.13 ab | 9.49 ± 0.16 ab | 200 | 200 | 200 | 400 |
| 8.0 | 9.53 ± 0.14 bc | 8.70 ± 0.14 d | 100 | 200 | 100 | 200 |
| Sample | Cholesterol Removal (%) | |
|---|---|---|
| 0.5% Oxgall | 0.5% TDCA | |
| Dead cells of L. plantarum EM | 39.58 ± 0.49 b | 34.67 ± 1.34 b |
| RRB | 8.87 ± 3.77 c | 7.77 ± 1.33 c |
| HNRB | 9.75 ± 1.06 c | 9.29 ± 3.10 c |
| HFRB | 67.58 ± 3.34 a | 44.93 ± 1.21 a |
| Indicator Strains | Antimicrobial Activity (AU/mL) | ||||
|---|---|---|---|---|---|
| RRB | HNRB | HFRB | MRS Culture Filtrate * | ||
| Molds | Aspergillus flavus ATCC 22546 | 0 | 0 | 200 | 200 |
| Aspergillus fumigatus ATCC 96918 | 0 | 0 | 400 | 600 | |
| Penicillium roqueforti ATCC 10110 | 0 | 0 | 100 | 100 | |
| Penicillium expansum ATCC 7861 | 0 | 0 | 0 | 100 | |
| Bacteria | Bacillus cereus ATCC 14579 | 0 | 0 | 200 | 300 |
| Escherichia coli O157:H7 ATCC 43895 | 0 | 0 | 200 | 200 | |
| Pseudomonas aeruginosa ATCC 29853 | 0 | 0 | 400 | 400 | |
| Salmonella enterica servoar. Typhi ATCC 14028 | 0 | 0 | 200 | 200 | |
| Sample | Phytic Acid (g/100 g) |
|---|---|
| RRB | 8.48 ± 0.47 a |
| HNRB | 7.93 ± 0.44 a |
| HFRB | 3.99 ± 0.26 b |
| Indicator Strains | RRB | HNRB | HFRB | HFRB-S |
|---|---|---|---|---|
| Sourness | 1.11 ± 0.33 d | 2.22 ± 0.44 c | 4.67 ± 0.50 a | 3.89 ± 0.78 b |
| Bitterness | 3.33 ± 0.71 b | 4.11 ± 0.33 a | 2.00 ± 0.00 c | 2.00 ± 0.00 c |
| Sweetness | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a |
| Hay smell | 5.00 ± 0.00 a | 4.00 ± 0.00 b | 1.67 ± 0.71 c | 1.67 ± 0.71 c |
| Pleasant flavor | 1.78 ± 0.83 c | 2.78 ± 0.83 b | 4.89 ± 0.33 a | 4.89 ± 0.33 a |
| Mouthfeel texture | 1.56 ± 0.53 c | 2.67 ± 0.50 b | 4.11 ± 0.60 a | 4.11 ± 0.33 a |
| Overall acceptability | 1.67 ± 0.50 c | 2.00 ± 0.00 c | 3.00 ± 0.50 b | 4.11 ± 0.60 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-H.; Chang, H.-C. Rice Bran Fermentation Using Lactiplantibacillus plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food. Foods 2021, 10, 978. https://doi.org/10.3390/foods10050978
Moon S-H, Chang H-C. Rice Bran Fermentation Using Lactiplantibacillus plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food. Foods. 2021; 10(5):978. https://doi.org/10.3390/foods10050978
Chicago/Turabian StyleMoon, Song-Hee, and Hae-Choon Chang. 2021. "Rice Bran Fermentation Using Lactiplantibacillus plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food" Foods 10, no. 5: 978. https://doi.org/10.3390/foods10050978
APA StyleMoon, S. -H., & Chang, H. -C. (2021). Rice Bran Fermentation Using Lactiplantibacillus plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food. Foods, 10(5), 978. https://doi.org/10.3390/foods10050978