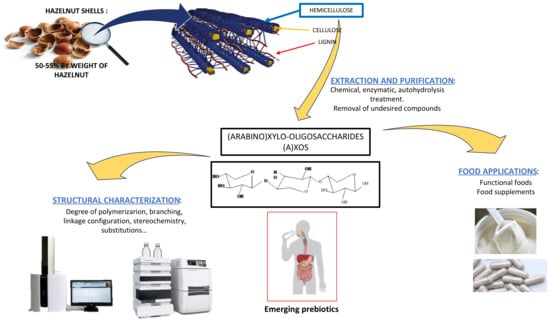
Potential Valorization of Hazelnut Shells through Extraction, Purification and Structural Characterization of Prebiotic Compounds: A Critical Review
Abstract
:1. Hazelnut and Circular Economy
2. Chemical Structure of (A)XOS
3. A(XOS): Emerging Prebiotics
3.1. Potential Health-Related Effects of (A)XOS
3.2. Recommended Dose and Exposure Assessment for (A)XOS
4. Extraction
4.1. Autohydrolysis Treatments
4.2. Enzymatic Treatment
5. Characterization of Obtained (A)XOS
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statistical Yearbooks—Material for the Nut and Dried Fruit Industry. Available online: https://www.nutfruit.org/industry/technical-resources (accessed on 4 April 2021).
- Köksal, A.I.; Artik, N.; Şimşek, A.; Güneş, N. Nutrient composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chem. 2006, 99, 509–515. [Google Scholar] [CrossRef]
- Savage, G.R.; McNeil, D.L. Chemical composition of hazelnuts (Corylus avellana L.) grown in New Zealand. Int. J. Food Sci. Nutr. 1998, 49, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Shahidi, F.; Liyanapathirana, C.M.; Ohshima, T. Turkish Tombul hazelnut (Corylus avellana L.). 1. Compositional characteristics. J. Agric. Food Chem. 2003, 51, 3790–3796. [Google Scholar] [CrossRef]
- McCance, R.; Widdowson, E. McCance and Widdowson’s The Composition of Foods, 5th ed.; The Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Lintas, C.; Cappelloni, M. Dietary fiber content of italian fruit and nuts. J. Food Compos. Anal. 1992, 5, 146–151. [Google Scholar] [CrossRef]
- Tunçil, Y.E. Dietary fibre profiles of Turkish Tombul hazelnut (Corylus avellana L.) and hazelnut skin. Food Chem. 2020, 316, 126338. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, F.; Akinci, I. Physical and nutritional properties of four major commercial Turkish hazelnut varieties. J. Food Eng. 2004, 63, 341–347. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic composition of hazelnut skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef]
- SOER 2015—The European Environment—State and Outlook 2015. Available online: https://www.eea.europa.eu/soer/2015 (accessed on 4 April 2021).
- Arslan, Y.; Takaç, S.; Eken-Saraçoĝlu, N. Kinetic study of hemicellulosic sugar production from hazelnut shells. Chem. Eng. J. 2012, 185, 23–28. [Google Scholar] [CrossRef]
- Demirbas, A. Furfural production from fruit shells by acid-catalyzed hydrolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2006, 28, 157–165. [Google Scholar] [CrossRef]
- Demirbaş, A. Estimating of structural composition of wood and non-wood biomass samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Surek, E.; Buyukkileci, A.O. Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohydr. Polym. 2017, 174, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Moure, A.; Parajó, J.C. Pretreatment of hazelnut shells as a key strategy for the solubilization and valorization of hemicelluloses into bioactive compounds. Agronomy 2020, 10, 760. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Bozan, B. Effect of Different Types of Thermochemical Pretreatment on the Enzymatic Hydrolysis and the Composition of Hazelnut Shells. Waste Biomass Valorization 2019, 11, 3739–3748. [Google Scholar] [CrossRef]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Amidon, T.E.; Liu, S. Water-based woody biorefinery. Biotechnol. Adv. 2009, 27, 542–550. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef]
- Şencan, A.; Karaboyacı, M.; Kılıç, M. Determination of lead(II) sorption capacity of hazelnut shell and activated carbon obtained from hazelnut shell activated with ZnCl2. Environ. Sci. Pollut. Res. 2015, 22, 3238–3248. [Google Scholar] [CrossRef]
- Cimino, G.; Passerini, A.; Toscano, G. Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res. 2000, 34, 2955–2962. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M.; Demirbas, E.; Öncel, M.S.; Sencan, S. Adsorption kinetic models applied to nickel ions on hazelnut shell activated carbons. Adsorpt. Sci. Technol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Demirbas, E.; Dizge, N.; Sulak, M.T.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Sert, S.; Çelik, A.; Tirtom, V.N. Removal of arsenic(III) ions from aqueous solutions by modified hazelnut shell. Desalin. Water Treat. 2017, 75, 115–123. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, K. Activated carbons prepared from hazelnut shells, walnut shells and peanut shells for high CO2 adsorption. Pol. J. Chem. Technol. 2017, 19, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Midilli, A.; Dogru, M.; Howarth, C.R.; Ayhan, T. Hydrogen production from hazelnut shell by applying air-blown downdraft gasification technique. Int. J. Hydrogen Energy 2001, 26, 29–37. [Google Scholar] [CrossRef]
- Midilli, A.; Rzayev, P.; Olgun, H.; Ayhan, T. Solar hydrogen production from hazelnut shells. Int. J. Hydrogen Energy 2000, 25, 723–732. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Berikten, D.; Kıvanç, M.; Bozan, B. Ethanol production from hazelnut shells through enzymatic saccharification and fermentation by low-temperature alkali pretreatment. Fuel 2017, 196, 280–287. [Google Scholar] [CrossRef]
- Pütün, A.E.; Özean, A.; Pütün, E. Pyrolysis of hazelnut shells in a fixed-bed tubular reactor: Yields and structural analysis of bio-oil. J. Anal. Appl. Pyrolysis 1999, 52, 33–49. [Google Scholar] [CrossRef]
- Çöpür, Y.; Güler, C.; Taşçioǧlu, C.; Tozluoǧlu, A. Incorporation of hazelnut shell and husk in MDF production. Bioresour. Technol. 2008, 99, 7402–7406. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Hanna, M.A. Valorization of hazelnut shells into natural antioxidants by ultrasound-assisted extraction: Process optimization and phenolic composition identification. J. Food Process Eng. 2018, 41, e12692. [Google Scholar] [CrossRef]
- Lopes, L.P.C.; Martins, J.; Esteves, B.; Lemos, L.T.D.E. New Products from Hazelnut Shell. In Proceedings of the ECOWOOD 2012—5th International Conference on Environmentally-Compatible Forest Products, Fernando Pessoa University, Oporto, Portugal, 5–7 September 2012. [Google Scholar]
- Charron, M. Exploiting the Potential of Hazelnut by-Products in a Confectionary Food Company. Ph.D. Thesis, University of Parma, Parma, Italy, 2020. [Google Scholar]
- Singh, R.D.; Banerjee, J.; Arora, A. Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. Fibre 2015, 5, 19–30. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Rennie, E.A.; Scheller, H.V. Xylan biosynthesis. Curr. Opin. Biotechnol. 2014, 26, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- de Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100184. [Google Scholar] [CrossRef]
- Dixit, Y.; Wagle, A.; Vakil, B. Patents in the Field of Probiotics, Prebiotics, Synbiotics: A Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 1000111. [Google Scholar] [CrossRef] [Green Version]
- de Farias, D.P.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.E.; Walker, W.A. Nutritional Impact of pre- and probiotics as Protective Gastrointestinal Organisms. Int. Semin. Pediatr. Gastroenterol. Nutr. 2002, 22, 107–138. [Google Scholar]
- Macfarlane, S.; Macfarlane, G.T.; Cummings, J.H. Review article: Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Geboes, K.P.; De Hertogh, G.; De Preter, V.; Luypaerts, A.; Bammens, B.; Evenepoel, P.; Ghoos, Y.; Geboes, K.; Rutgeerts, P.; Verbeke, K. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br. J. Nutr. 2006, 96, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar]
- Mäkeläinen, H.; Juntunen, M.; Hasselwander, O. Prebiotic Potential of Xylo-Oligosaccharides. In Prebiotics and Probiotics Science and Technology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 245–258. [Google Scholar]
- Saville, B.A.; Saville, S.H. Functional Attributes and Health Benefits of Novel Prebiotic Oligosaccharides Derived from Xylan, Arabinan, and Mannan. In Prebiotics and Probiotics—Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtin, C.M.; Broekaert, W.F.; Swennen, K. Occurrence of arabinoxylo-oligosaccharides and arabinogalactan peptides in beer. J. Am. Soc. Brew. Chem. 2009, 62, 112–117. [Google Scholar] [CrossRef]
- Kabel, M.A.; Kortenoeven, L.; Schols, H.A.; Voragen, A.G.J. In vitro fermentability of differently substituted xylo-oligosaccharides. J. Agric. Food Chem. 2002, 50, 6205–6210. [Google Scholar] [CrossRef]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; Van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; Van De Wiele, T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.M.; Fahey, G.C.; Wolf, B.W. Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, pH and Microflora in Rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef]
- Van Craeyveld, V.; Swennen, K.; Dornez, E.; Van de Wiele, T.; Marzorati, M.; Verstraete, W.; Delaedt, Y.; Onagbesan, O.; Decuypere, E.; Buyse, J.; et al. Structurally Different Wheat-Derived Arabinoxylooligosaccharides Have Different Prebiotic and Fermentation Properties in Rats. J. Nutr. 2008, 138, 2348–2355. [Google Scholar] [CrossRef]
- Vardakou, M.; Nueno Palop, C.; Gasson, M.; Narbad, A.; Christakopoulos, P. In vitro three-stage continuous fermentation of wheat arabinoxylan fractions and induction of hydrolase activity by the gut microflora. Int. J. Biol. Macromol. 2007, 41, 584–589. [Google Scholar] [CrossRef]
- Kjølbæk, L.; Benítez-Páez, A.; Gómez del Pulgar, E.M.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Finegold, S.M.; Li, Z.; Summanen, P.H.; Downes, J.; Thames, G.; Corbett, K.; Dowd, S.; Krak, M.; Heber, D. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. 2014, 5, 436–445. [Google Scholar] [CrossRef]
- Lin, S.H.; Chou, L.M.; Chien, Y.W.; Chang, J.S.; Lin, C.I. Prebiotic Effects of Xylooligosaccharides on the Improvement of Microbiota Balance in Human Subjects. Gastroenterol. Res. Pract. 2016, 2016, 5789232. [Google Scholar] [CrossRef] [Green Version]
- Gobinath, D.; Madhu, A.N.; Prashant, G.; Srinivasan, K.; Prapulla, S.G. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br. J. Nutr. 2010, 104, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenashri, B.R.; Muralikrishna, G. In vitro anti-oxidant activity of xylo-oligosaccharides derived from cereal and millet brans—A comparative study. Food Chem. 2011, 126, 1475–1481. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Wang, C.; Sun, B. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohydr. Polym. 2011, 86, 1192–1197. [Google Scholar] [CrossRef]
- Na, M.; Kim, W. Effects of xylooligosaccharide intake on fecal Bifidobacteria, lactic acid and lipid metabolism in Korean young women. Korean J. Nutr. 2007, 40, 154–161. [Google Scholar]
- Chung, Y.C.; Hsu, C.K.; Ko, C.Y.; Chan, Y.C. Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr. Res. 2007, 27, 756–761. [Google Scholar] [CrossRef]
- Iino, T.; Nishijima, Y.; Sawada, S.; Sasaki, H.; Harada, H.; Suwa, Y.; Kiso, Y. Improvement of Constipation by a Small Amount of Xylooligosaccharides Ingestion in Adult Women. J. Jpn. Assoc. Diet. Fiber Res. 1997, 1, 19–24. [Google Scholar]
- Yang, J.; Summanen, P.H.; Henning, S.M.; Hsu, M.; Lam, H.; Huang, J.; Tseng, C.H.; Dowd, S.E.; Finegold, S.M.; Heber, D.; et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study. Front. Physiol. 2015, 6, 216. [Google Scholar] [CrossRef] [Green Version]
- Aachary, A.A.; Prapulla, S.G. Value addition to corncob: Production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour. Technol. 2009, 100, 991–995. [Google Scholar] [CrossRef]
- Cloetens, L.; Broekaert, W.F.; Delaedt, Y.; Ollevier, F.; Courtin, C.M.; Delcour, J.A.; Rutgeerts, P.; Verbeke, K. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: A randomised, placebo-controlled cross-over study. Br. J. Nutr. 2010, 103, 703–713. [Google Scholar] [CrossRef]
- Damen, B.; Cloetens, L.; Broekaert, W.F.; François, I.; Lescroart, O.; Trogh, I.; Arnaut, F.; Welling, G.W.; Wijffels, J.; Delcour, J.A.; et al. Consumption of Breads Containing In Situ–Produced Arabinoxylan Oligosaccharides Alters Gastrointestinal Effects in Healthy Volunteers. J. Nutr. 2012, 142, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Páez, A.; Kjølbæk, L.; Gómez del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.-F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans. mSystems 2019, 4, e00209-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Evenepoel, P.; Hamer, H.; Houben, E.; Windey, K.; Welling, G.W.; et al. Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: A double-blind, randomised, placebo-controlled, cross-over trial. Br. J. Nutr. 2012, 108, 2229–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendis, M.; Simsek, S. Arabinoxylans and human health. Food Hydrocoll. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Smith, C. The molecular structure features-immune stimulatory activity of arabinoxylans derived from the pentosan faction of wheat flour. J. Cereal Sci. 2015, 62, 81–86. [Google Scholar] [CrossRef]
- Boll, E.V.J.; Ekström, L.M.N.K.; Courtin, C.M.; Delcour, J.A.; Nilsson, A.C.; Björck, I.M.E.; Östman, E.M. Effects of wheat bran extract rich in arabinoxylan oligosaccharides and resistant starch on overnight glucose tolerance and markers of gut fermentation in healthy young adults. Eur. J. Nutr. 2016, 55, 1661–1670. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of xylo-oligosaccharides (XOS) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, e05361. [Google Scholar]
- Gao, Y.; Zhang, S.; Li, C.; Xiao, L.; Shen, J.; Yin, J. Acute and subchronic toxicity of xylo-oligosaccharide in mice and rats. Toxicol. Mech. Methods 2012, 22, 605–610. [Google Scholar] [CrossRef]
- Xiao, L.; Ning, J.; Xu, G. Application of Xylo-oligosaccharide in modifying human intestinal function. Afr. J. Microbiol. Res. 2012, 6, 2116–2119. [Google Scholar]
- Kobayashi, T.; Fujikawa, S.; Koga, K.; Okazaki, M. Effect of Xylooligosaccharides on Feces of Men. Nippon Nogeikagaku Kaishi 1991, 48, 19–29. [Google Scholar]
- Commission Implementing Regulation (EU) 2018/1648. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1648&from=EN (accessed on 10 April 2021).
- Commission Implementing Regulation (EU) 2020/916. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/916/oj (accessed on 10 April 2021).
- Cloetens, L.; De Preter, V.; Rutgeerts, P.; Verbeke, K.; Swennen, K.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A. Dose-Response Effect of Arabinoxylooligosaccharides on Gastrointestinal Motility and on Colonic Bacterial Metabolism in Healthy Volunteers. J. Am. Coll. Nutr. 2008, 27, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) 2017/2470. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 10 April 2021).
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. Lignocellulosic conversion into value-added products: A review. Process Biochem. 2020, 89, 110–133. [Google Scholar] [CrossRef]
- Gírio, F.M.; Carvalheiro, F.; Duarte, L.C.; Bogel-ŁUkasik, R. Deconstruction of the hemicellulose fraction from lignocellulosic materials into simple sugars. In D-Xylitol: Fermentative Production, Application and Commercialization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–37. [Google Scholar]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Oliva, J.M.; Ballesteros, I.; Ballesteros, M. Changes in various physical/chemical parameters of Pinus pinaster wood after steam explosion pretreatment. Biomass Bioenergy 2003, 25, 301–308. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; de Oliva Neto, P.; da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Moniz, P. Hydrothermal/Liquid Hot Water Pretreatment (Autohydrolysis): A Multipurpose Process for Biomass Upgrading. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–347. [Google Scholar]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Ebringerová, A.; Montané, D. Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohydr. Polym. 2007, 69, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef] [Green Version]
- Nabarlatz, D.; Farriol, X.; Montané, D. Autohydrolysis of almond shells for the production of xylo-oligosaccharides: Product characteristics and reaction kinetics. Ind. Eng. Chem. Res. 2005, 44, 7746–7755. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef]
- Rostro, M.; Sánchez-González, M.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Non-isothermal autohydrolysis of nixtamalized maize pericarp: Production of nutraceutical extracts. LWT Food Sci. Technol. 2014, 58, 550–556. [Google Scholar] [CrossRef]
- Immerzeel, P.; Falck, P.; Galbe, M.; Adlercreutz, P.; Nordberg Karlsson, E.; Stålbrand, H. Extraction of water-soluble xylan from wheat bran and utilization of enzymatically produced xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp. LWT Food Sci. Technol. 2014, 56, 321–327. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Hames, B.R.; Thomas, S.R.; Sluiter, A.D.; Roth, C.J.; Templeton, D.W. Rapid Biomass Analysis. In Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2003; Volume 105, pp. 5–16. [Google Scholar]
- Vegas, R.; Luque, S.; Alvarez, J.R.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Membrane-Assisted Processing of Xylooligosaccharide-Containing Liquors. J. Agric. Food Chem. 2006, 54, 5430–5436. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Alonso, J.L.; Parajó, J.C.; Yáñez, R. Valorization of peanut shells: Manufacture of bioactive oligosaccharides. Carbohydr. Polym. 2018, 183, 21–28. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Torras, C.; Garcia-Valls, R.; Montané, D. Purification of xylo-oligosaccharides from almond shells by ultrafiltration. Sep. Purif. Technol. 2007, 53, 235–243. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Jacobs, A.; Palm, M.; Zacchi, G.; Dahlman, O. Isolation and characterization of water-soluble hemicelluloses from flax shive. Carbohydr. Res. 2003, 338, 1869–1876. [Google Scholar] [CrossRef]
- Katapodis, P.; Vardakou, M.; Kalogeris, E.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Enzymic production of a feruloylated oligosaccharide with antioxidant activity from wheat flour arabinoxylan. Eur. J. Nutr. 2003, 42, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Chapla, D.; Pandit, P.; Shah, A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour. Technol. 2012, 115, 215–221. [Google Scholar] [CrossRef]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef]
- Mathew, S.; Karlsson, E.N.; Adlercreutz, P. Extraction of soluble arabinoxylan from enzymatically pretreated wheat bran and production of short xylo-oligosaccharides and arabinoxylo-oligosaccharides from arabinoxylan by glycoside hydrolase family 10 and 11 endoxylanases. J. Biotechnol. 2017, 260, 53–61. [Google Scholar] [CrossRef]
- Juvonen, M.; Kotiranta, M.; Jokela, J.; Tuomainen, P.; Tenkanen, M. Identification and structural analysis of cereal arabinoxylan-derived oligosaccharides by negative ionization HILIC-MS/MS. Food Chem. 2019, 275, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Falck, P.; Aronsson, A.; Grey, C.; Stålbrand, H.; Nordberg Karlsson, E.; Adlercreutz, P. Production of arabinoxylan-oligosaccharide mixtures of varying composition from rye bran by a combination of process conditions and type of xylanase. Bioresour. Technol. 2014, 174, 118–125. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Prather, K.L.J.; Rodrigues, L.R. Single-step production of arabino-xylooligosaccharides by recombinant Bacillus subtilis 3610 cultivated in brewers’ spent grain. Carbohydr. Polym. 2018, 199, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, O.; Erdogan, K.; Bostanci, S. Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr. Res. 2009, 344, 660–666. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Alonso, J.L.; Sáez, F.; Negro, M.J.; Gullón, B. Xylooligosaccharides from steam-exploded barley straw: Structural features and assessment of bifidogenic properties. Food Bioprod. Process. 2020, 124, 131–142. [Google Scholar] [CrossRef]
- Amicucci, M.J.; Galermo, A.G.; Nandita, E.; Vo, T.T.T.; Liu, Y.; Lee, M.; Xu, G.; Lebrilla, C.B. A rapid-throughput adaptable method for determining the monosaccharide composition of polysaccharides. Int. J. Mass Spectrom. 2019, 438, 22–28. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Liu, X.; Liu, B.; Cao, H.; Yu, H.; Sarker, S.D.; Nahar, L.; Xiao, J. Functional properties, structural studies and chemo-enzymatic synthesis of oligosaccharides. Trends Food Sci. Technol. 2017, 66, 135–145. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhao, X.; Pu, J.H.; Luan, X.H. Influences of acidic reaction and hydrolytic conditions on monosaccharide composition analysis of acidic, neutral and basic polysaccharides. Carbohydr. Polym. 2016, 143, 296–300. [Google Scholar] [CrossRef]
- Willför, S.; Pranovich, A.; Tamminen, T.; Puls, J.; Laine, C.; Suurnäkki, A.; Saake, B.; Uotila, K.; Simolin, H.; Hemming, J.; et al. Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides—A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind. Crops Prod. 2009, 29, 571–580. [Google Scholar] [CrossRef]
- Zaia, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef]
- Amicucci, M.J.; Nandita, E.; Lebrilla, C.B. Function without Structures: The Need for In-Depth Analysis of Dietary Carbohydrates. J. Agric. Food Chem. 2019, 67, 4418–4424. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Hou, J.; Long, H.; Wang, B.; Guo, D.; Lei, M.; Wu, W. Gastrodia elata blume polysaccharides: A review of their acquisition, analysis, modification, and pharmacological activities. Molecules 2019, 24, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechelke, M.; Herlet, J.; Benz, J.P.; Schwarz, W.H.; Zverlov, V.V.; Liebl, W.; Kornberger, P. HPAEC-PAD for oligosaccharide analysis—novel insights into analyte sensitivity and response stability. Anal. Bioanal. Chem. 2017, 409, 7169–7181. [Google Scholar] [CrossRef] [PubMed]
- Alyassin, M.; Campbell, G.M.; Masey O’Neill, H.; Bedford, M.R. Simultaneous determination of cereal monosaccharides, xylo- and arabinoxylo-oligosaccharides and uronic acids using HPAEC-PAD. Food Chem. 2020, 315, 126221. [Google Scholar] [CrossRef] [PubMed]
- Pastell, H.; Tuomainen, P.; Virkki, L.; Tenkanen, M. Step-wise enzymatic preparation and structural characterization of singly and doubly substituted arabinoxylo-oligosaccharides with non-reducing end terminal branches. Carbohydr. Res. 2008, 343, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhao, X.; Xiao, L.; Zhao, H. Development and validation of a HILIC-ELSD method for simultaneous analysis of non-substituted and acetylated xylo-oligosaccharides. J. Pharm. Biomed. Anal. 2017, 139, 232–237. [Google Scholar] [CrossRef]
- Mafei, T.D.T.; Neto, F.S.P.P.; Peixoto, G.; de Baptista Neto, Á.; Monti, R.; Masarin, F. Extraction and Characterization of Hemicellulose from Eucalyptus By-product: Assessment of Enzymatic Hydrolysis to Produce Xylooligosaccharides. Appl. Biochem. Biotechnol. 2020, 190, 197–217. [Google Scholar] [CrossRef]
- Ding, C.; Li, M.; Hu, Y. High-activity production of xylanase by Pichia stipitis: Purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 2018, 117, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2011, 399, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Bowman, M.J.; Dien, B.S.; Vermillion, K.E.; Mertens, J.A. Structural characterization of (1→2)-β-xylose-(1→3)-α-arabinose-containing oligosaccharide products of extracted switchgrass (Panicum virgatum, L.) xylan after exhaustive enzymatic treatment with α-arabinofuranosidase and β-endo-xylanase. Carbohydr. Res. 2014, 398, 63–71. [Google Scholar] [CrossRef]
- Suzuki, S. Recent developments in liquid chromatography and capillary electrophoresis for the analysis of glycoprotein glycans. Anal. Sci. 2013, 29, 1117–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Freeman, S.; Corey, M.; German, J.B.; Barile, D. Chemical Characterization of Potentially Prebiotic Oligosaccharides in Brewed Coffee and Spent Coffee Grounds. J. Agric. Food Chem. 2017, 65, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Leijdekkers, A.G.M.; Sanders, M.G.; Schols, H.A.; Gruppen, H. Characterizing plant cell wall derived oligosaccharides using hydrophilic interaction chromatography with mass spectrometry detection. J. Chromatogr. A 2011, 1218, 9227–9235. [Google Scholar] [CrossRef]
- Quéméner, B.; Ordaz-Ortiz, J.J.; Saulnier, L. Structural characterization of underivatized arabino-xylo-oligosaccharides by negative-ion electrospray mass spectrometry. Carbohydr. Res. 2006, 341, 1834–1847. [Google Scholar] [CrossRef]
- Xiao, X.; Wen, J.Y.; Wang, Y.Y.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. NMR and ESI–MS spectrometry characterization of autohydrolysis xylo-oligosaccharides separated by gel permeation chromatography. Carbohydr. Polym. 2018, 195, 303–310. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Coimbra, M.A.; Domingues, P.; Ferrer-Correia, A.J.; Rosário, M.; Domingues, M. Structural characterisation of underivatised olive pulp xylo-oligosaccharides by mass spectrometry using matrix-assisted laser desorption/ionisation and electrospray ionisation. Rapid Commun. Mass Spectrom. 2002, 16, 2124–2132. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Pintado, M.; Gomes, A.M.; Alonso, J.L.; Parajó, J.C. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J. Funct. Foods 2014, 6, 438–449. [Google Scholar] [CrossRef]
- Rivas, S.; Santos, V.; Parajó, J.C. Aqueous fractionation of hardwood: Selective glucuronoxylan solubilisation and purification of the reaction products. J. Chem. Technol. Biotechnol. 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Ruiz, E.; Gullón, B.; Moura, P.; Carvalheiro, F.; Eibes, G.; Cara, C.; Castro, E. Bifidobacterial growth stimulation by oligosaccharides generated from olive tree pruning biomass. Carbohydr. Polym. 2017, 169, 149–156. [Google Scholar] [CrossRef]
- Kabel, M.A.; Carvalheiro, F.; Garrote, G.; Avgerinos, E.; Koukios, E.; Parajó, J.C.; Gírio, F.M.; Schols, H.A.; Voragen, A.G.J. Hydrothermally treated xylan rich by-products yield different classes of xylo-oligosaccharides. Carbohydr. Polym. 2002, 50, 47–56. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chu, X.; Zhao, Z.X.; He, X.S.; Guo, Y.L. Analysis of low molecular weight compounds by MALDI-FTICR-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1166–1179. [Google Scholar] [CrossRef]
- Lee, H.; An, H.J.; Lerno, L.A.; German, J.B.; Lebrilla, C.B. Rapid profiling of bovine and human milk gangliosides by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Int. J. Mass Spectrom. 2011, 305, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jänis, J.; Hakanpää, J.; Hakulinen, N.; Ibatullin, F.M.; Hoxha, A.; Derrick, P.J.; Rouvinen, J.; Vainiotalo, P. Determination of thioxylo-oligosaccharide binding to family 11 xylanases using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and X-ray crystallography. FEBS J. 2005, 272, 2317–2333. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lebrilla, C.B. Application of Fourier transform ion cyclotron resonance mass spectrometry to oligosaccharides. Mass Spectrom. Rev. 2005, 24, 232–264. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Hahm, H.S.; Seeberger, P.H.; Pagel, K. Identification of carbohydrate anomers using ion mobility-mass spectrometry. Nature 2015, 526, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Schulz, B.L.; Ferro, V. Applications of ion mobility-mass spectrometry in carbohydrate chemistry and glycobiology. Molecules 2018, 23, 2557. [Google Scholar] [CrossRef] [Green Version]
- Both, P.; Green, A.P.; Gray, C.J.; Šardzík, R.; Voglmeir, J.; Fontana, C.; Austeri, M.; Rejzek, M.; Richardson, D.; Field, R.A.; et al. Discrimination of epimeric glycans and glycopeptides using IM-MS and its potential for carbohydrate sequencing. Nat. Chem. 2014, 6, 65–74. [Google Scholar] [CrossRef]


| XOS/AXOS | Pretreatment/Extraction | Hydrolysis | Substrate | (A)XOS Production Yield | DP | Reference |
|---|---|---|---|---|---|---|
| xos | alkali | enzymatic hydrolysis | corncobs | 81% of the original xylan | 2–7 | [77] |
| xos | acid | enzymatic hydrolysis | corncobs | 52% of the original xylan | 2–7 | |
| xos | steam explosion | enzymatic hydrolysis | corncobs | 77% of the original xylan | 2–7 | |
| xos | alkali | acid hydrolysis (H2SO4) | tobacco stalk | 13% of the original xylan | 1–6 | [126] |
| xos | alkali | acid hydrolysis (H2SO4) | cotton stalk | 7.5% of the original xylan | 1–6 | |
| xos | alkali | acid hydrolysis (H2SO4) | sunflower stalk | 12.6% of the original xylan | 1–6 | |
| xos | alkali | acid hydrolysis (H2SO4) | wheat straw | 10.2% of the original xylan | 1–6 | |
| xos | alkali | enzymatic hydrolysis | corncob | 17.9% of raw material | 2–5 | [119] |
| xos | hydrothermal, 210 °C 15 min | - | hazelnut shell | 73.7% of the original xylan | 3–16 | [15] |
| xos | hydrothermal, 190 °C, 5 min | - | hazelnut shell | 62% of the original xylan | 2–>6 | [14] |
| xos | hydrothermal, 190 °C, 19 min | - | almond shell | 63% of the original xylan | n.d | [105] |
| xos | hydrothermal, 179 °C, 23 min | - | corncobs | 60% of the original xylan | n.d | [103] |
| xos | hydrothermal, 179 °C, 23 min | - | almond shell | 55% of the original xylan | n.d | |
| xos | hydrothermal, 179 °C, 23 min | - | olive stones | 43% of the original xylan | n.d | |
| xos | hydrothermal, 179 °C, 23 min | - | rice husks | 30% of the original xylan | n.d | |
| xos | hydrothermal, 179 °C, 23 min | - | wheat straw | 43% of the original xylan | n.d | |
| xos | hydrothermal, 179 °C, 23 min | - | barley straw | 43% of the original xylan | n.d | |
| xos | hydrothermal, 210 °C, until reaching temperature then fast cooling | - | Palm empty fruit bunches fibre | 63% of the original xylan | 5–40 | [104] |
| xos | hydrothermal, 121 °C, 60min | enzymatic hydrolysis | oil palm frond fibres | 15% of raw material | 1–4 | [120] |
| xos | hydrothermal, 200 °C, 5 min | enzymatic hydrolysis | almond shell | 54.5% of the original xylan | 76.8% low DP | [114] |
| xos | steam explosion | enzymatic hydrolysis | barley straw | 60.2% of the original xylan | 4–9 | [127] |
| axos | hydrothermal, 210 °C, 2 min | - | brewery spent grain | 43.4% of the original AX | 8–18 | [106] |
| axos | hydrothermal, 207 °C, until reaching temperature then fast cooling | - | nixtamalized maize pericarp | 39.6% of raw material (based on dm) | n.d | [107] |
| axos | hydrothermal, 185 °C, 10 min | enzymatic hydrolysis | wheat bran | 59% of the original AX | 2–5 | [108] |
| axos | hydrothermal, 215 °C, until reaching temperature then fast cooling | - | wheat straw | 64% of the original AX | n.d | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuso, A.; Risso, D.; Rosso, G.; Rosso, F.; Manini, F.; Manera, I.; Caligiani, A. Potential Valorization of Hazelnut Shells through Extraction, Purification and Structural Characterization of Prebiotic Compounds: A Critical Review. Foods 2021, 10, 1197. https://doi.org/10.3390/foods10061197
Fuso A, Risso D, Rosso G, Rosso F, Manini F, Manera I, Caligiani A. Potential Valorization of Hazelnut Shells through Extraction, Purification and Structural Characterization of Prebiotic Compounds: A Critical Review. Foods. 2021; 10(6):1197. https://doi.org/10.3390/foods10061197
Chicago/Turabian StyleFuso, Andrea, Davide Risso, Ginevra Rosso, Franco Rosso, Federica Manini, Ileana Manera, and Augusta Caligiani. 2021. "Potential Valorization of Hazelnut Shells through Extraction, Purification and Structural Characterization of Prebiotic Compounds: A Critical Review" Foods 10, no. 6: 1197. https://doi.org/10.3390/foods10061197
APA StyleFuso, A., Risso, D., Rosso, G., Rosso, F., Manini, F., Manera, I., & Caligiani, A. (2021). Potential Valorization of Hazelnut Shells through Extraction, Purification and Structural Characterization of Prebiotic Compounds: A Critical Review. Foods, 10(6), 1197. https://doi.org/10.3390/foods10061197