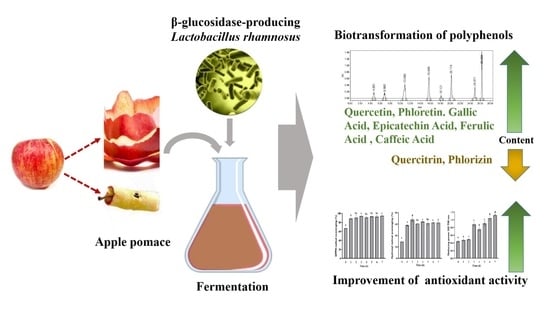
Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains and Culture Conditions
2.3. β-Glucosidase Activity
2.4. Effects of Culture Conditions on β-Glucosidase Production
2.5. Fermentation of Apple Pomace
2.6. Total Phenolic Content (TPC)
2.7. Quantification of Polyphenols in Fermentation Apple Pomace by HPLC
2.8. Determination of Antioxidant Activities
2.8.1. DPPH Radical Scavenging Activity
2.8.2. Hydroxyl Radical Scavenging Activity
2.8.3. Reducing Power Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Screening for Strains Producing β-Glucosidase
3.2. TPC during Fermentation
3.3. Phenolic Profiles during Fermentation
3.4. Changes in Antioxidant Activities during Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Papanikolaou, S.; Kwan, T.H.; Luque, R. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: A global perspective. Biofuels Bioprod. Biorefin. 2014, 8, 686–715. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Jung, J.; Cavender, G.; Zhao, Y. Impingement drying for preparing dried apple pomace flour and its fortification in bakery and meat products. J. Food Sci. Technol. 2015, 52, 5568–5578. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple pomace as potential source of natural active compounds. Planta Med. 2017, 84, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Tu, S.H.; Chen, L.C.; Ho, Y.S. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. J. Food Drug Anal. 2017, 34, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Nieuwenhove, C.V.; Mozzi, F.; Alberto, J.; Burgos, S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2020, e109774. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Wu, J.C.; Huang, Q.; Shahidi, F.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, e109064. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2020, 339, e127859. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, E.A.; Kim, D.H.; Shin, K.S. The bioconversion of red ginseng ethanol extract into compound K by Saccharomyces Cerevisiae HJ-014. Mycobiology 2014, 42, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; Pascual-Teresa, S.D. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- He, J.; Dong, Y.; Liu, X.; Wan, Y.; Gu, T.; Zhou, X.; Liu, M. Comparison of chemical compositions, antioxidant, and anti-photoaging activities of Paeonia suffruticosa flowers at different flowering stages. Antioxidants 2019, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Yao, R.; Wong, C.B.; Nakamura, K.; Mitsuyama, E.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Xiao, J.Z. Bifidobacterium breve MCC1274 with glycosidic activity enhances in vivo isoflavone bioavailability. Benef. Microbes 2019, 10, 521–531. [Google Scholar] [CrossRef]
- Modrackova, N.; Vlkova, E.; Tejnecky, V.; Schwab, C.; Neuzil-Bunesova, V. Bifidobacterium β-Glucosidase activity and fermentation of dietary plant glucosides is species and strain specific. Microorganisms 2020, 8, 839. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process. Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Rio, D.D.; Galaverna, G.; Lazzi, G. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2018, 276, 692–699. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotification with Lactobacillus plantarum MCC 2974 on quality of Sohiong juice. LWT 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Kamiloglu, S.; Demirci Cekic, S.; Sozgen Baskan, K.; Avan, A.N.; Uzunboy, S.; Apak, R. Antioxidant Activity and Capacity Measurement. Plant. Antioxid. Health 2020, 1–66. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, e6520. [Google Scholar] [CrossRef] [PubMed]
- Krawitzky, M.; Arias, E.; Peiro, J.M.; Negueruela, A.I.; Val, J.; Oria, R. Determination of color, antioxidant activity, and phenolic profile of different fruit tissue of Spanish “Verde Doncella” apple cultivar. Int. J. Food Prop. 2014, 17, 2298–2311. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, e112250. [Google Scholar] [CrossRef]
- Cheng, J.R.; Liu, X.M.; Chen, Z.Y.; Zhang, Y.S.; Zhang, Y.H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Saa, D.L.T.; Gianotti, A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Yuri, J.A.; Neira, A.; Quilodran, A.; Motomura, Y.; Palomo, I. Antioxidant activity and total phenolics concentration in apple peel and flesh is determined by cultivar and agroclimatic growing regions in Chile. J. Food Agric. Environ. 2009, 7, 513–517. [Google Scholar]






| Fermentation Time (d) | Phenolic Acids | Flavonoid Glycosides | Flavonoid Aglycones | |||||
|---|---|---|---|---|---|---|---|---|
| Gallic Acid | Epicatechin Acid | Caffeic Acid | Ferulic Acid | Quercitrin | Phlorizin | Quercetin | Phloretin | |
| 0 | 0.10 ± 0.08 a | 0.40 ± 0.02 a | 0.62 ± 0.03 a | 0.83 ± 0.05 a | 34.11 ± 0.04 f | 3.43 ± 0.03 g | 0.54 ± 0.04 a | 1.05 ± 0.05 a |
| 1 | 0.15 ± 0.02 a | 1.27 ± 0.03 b | 0.98 ± 0.04 b | 2.76 ± 0.06 d | 35.57 ± 0.02 g | 3.43 ± 0.04 g | 0.54 ± 0.06 a | 1.10 ± 0.02 a |
| 2 | 1.26 ± 0.11 b | 1.50 ± 0.04 c | 0.98 ± 0.03 b | 2.93 ± 0.05 e | 35.61 ± 0.05 g | 3.04 ± 0.06 f | 0.55 ± 0.03 a | 1.35 ± 0.06 b |
| 3 | 2.00 ± 0.22 c | 1.93 ± 0.04 e | 1.12 ± 0.07 c | 2.71 ± 0.02 d | 32.28 ± 0.06 e | 1.87 ± 0.04 e | 3.22 ± 0.04 b | 2.38 ± 0.03 c |
| 4 | 1.37 ± 0.02 b | 1.95 ± 0.08 e | 1.00 ± 0.05b c | 2.73 ± 0.05 d | 31.60 ± 0.02 d | 1.67 ± 0.02 d | 5.44 ± 0.02 c | 2.46 ± 0.03 c |
| 5 | 1.99 ± 0.08 c | 1.69 ± 0.04 d | 1.08 ± 0.05b c | 1.71 ± 0.03 b | 30.28 ± 0.02 c | 1.38 ± 0.05 c | 5.45 ± 0.01 c | 2.61 ± 0.02 d |
| 6 | 2.08 ± 0.18 c | 2.29 ± 0.10 f | 1.05 ± 0.06b c | 2.63 ± 0.07 d | 28.99 ± 0.07 b | 1.21 ± 0.01 b | 6.00 ± 0.01 d | 2.69 ± 0.04 d |
| 7 | 2.05 ± 0.10 c | 2.69 ± 0.06 g | 1.01 ± 0.03b c | 2.45 ± 0.05 c | 27.36 ± 0.04 a | 0.97 ± 0.03 a | 6.40 ± 0.03 e | 3.09 ± 0.04 e |
| DPPH-SA | OH-SA | RPA | |
|---|---|---|---|
| Gallic acid | 0.765 * | 0.649 | 0.892 ** |
| Epicatechin acid | 0.863 ** | 0.766 * | 0.876 ** |
| Caffeic acid | 0.971 ** | 0.896 ** | 0.607 |
| Ferulic acid | 0.781 * | 0.865 ** | 0.197 |
| Quercetin | 0.590 | 0.444 | 0.927 ** |
| Phloretin | 0.681 | 0.517 | 0.972 ** |
| Quercitrin | −0.455 | −0.267 | −0.956 ** |
| Phlorizin | −0.669 | −0.514 | −0.969 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. https://doi.org/10.3390/foods10061343
Liu L, Zhang C, Zhang H, Qu G, Li C, Liu L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods. 2021; 10(6):1343. https://doi.org/10.3390/foods10061343
Chicago/Turabian StyleLiu, Lihua, Chenyi Zhang, Huimin Zhang, Guoqiang Qu, Chun Li, and Libo Liu. 2021. "Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08" Foods 10, no. 6: 1343. https://doi.org/10.3390/foods10061343
APA StyleLiu, L., Zhang, C., Zhang, H., Qu, G., Li, C., & Liu, L. (2021). Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods, 10(6), 1343. https://doi.org/10.3390/foods10061343