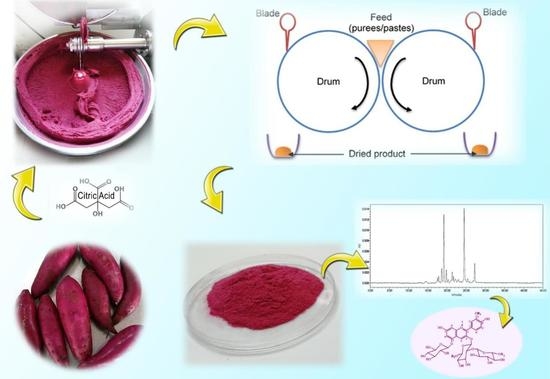
Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Drum Drying Operation
2.4. Experimental Design and Data Analysis
2.5. Physicochemical Analyses of the PSPP
2.5.1. Determination of Moisture Content
2.5.2. Determination of Water Activity
2.5.3. Determination of Color
2.5.4. Determination of the Water Solubility Index and Water Absorption Capacity
2.5.5. Thermal Properties
2.5.6. Determination of pH
2.6. Determination of the Antioxidant Activity
2.6.1. Extraction of Antioxidants
2.6.2. UV Absorption Spectra Scanning
2.6.3. High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS)
2.6.4. Determination of the Total Anthocyanin Content
2.6.5. Determination of the Total Flavonoid Content
2.6.6. Determination of the Total Phenolic Content
2.6.7. Determination of DPPH Radical Scavenging Activity
2.6.8. Determination of Ferric Reducing Antioxidant Power
2.7. Statistical Analysis and Validation of the Model
3. Results
3.1. UV Absorption Spectra of the PSPP
3.2. Response Surface Analysis
3.3. Model Adequacy
3.4. Moisture Content and Water Activity
3.5. Color
3.6. Total Anthocyanin Content
3.7. Antioxidant Activity
3.8. Water Solubility Index and Water Absorption Capacity
3.9. Optimisation of Drum Drying Process Parameters for Production of PSPP
3.10. Verification of the Final Reduced Models
3.11. HPLC-MSn Analyses of Anthocyanins
3.12. HPLC Quantification of PSPP Anthocyanins
3.13. Thermal Properties of the Optimized PSP Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konczak-Islam, I.; Yoshimoto, M.; Hou, D.X.; Terahara, N.; Yamakawa, O. Potential Chemopreventive Properties of Anthocyanin-Rich Aqueous Extracts from in Vitro Produced Tissue of Sweetpotato (Ipomoea batatas L.). J. Agric. Food Chem. 2003, 51, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- International Potato Center. Facts and Figures about Sweetpotato. Available online: http://cipotato.org/sweetpotato (accessed on 20 May 2019).
- Zhu, F.; Cai, Y.Z.; Yang, X.; Ke, J.; Corke, H. Anthocyanins, Hydroxycinnamic Acid Derivatives, and Antioxidant Activity in Roots of Different Chinese Purple-Fleshed Sweetpotato Genotypes. J. Agric. Food Chem. 2010, 58, 7588–7596. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Gras, C.C.; Nemetz, N.; Carle, R.; Schweiggert, R.M. Anthocyanins from Purple Sweet Potato (Ipomoea batatas (L.) Lam.) and Their Color Modulation by the Addition of Phenolic Acids and Food-Grade Phenolic Plant Extracts. Food Chem. 2017, 235, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Nevara, G.A.; Yea, C.S.; Karim, R.; Muhammad, K.; Mohd Ghazali, H. Effects of Moist-Heat Treatments on Color Improvement, Physicochemical, Antioxidant, and Resistant Starch Properties of Drum-Dried Purple Sweet Potato Powder. J. Food Process. Eng. 2019, 42, e12951. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Na Kim, S.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin Changes in the Korean Purple-Fleshed Sweet Potato, Shinzami, as Affected by Steaming and Baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different PH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, H.; Dong, N.; Zhao, G. Degradation Kinetics of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) as Affected by Ascorbic Acid. Food Sci. Biotechnol. 2014, 23, 89–96. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical Constituents and Health Effects of Sweet Potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef]
- Li, J.; Li, X.D.; Zhang, Y.; Zheng, Z.D.; Qu, Z.Y.; Liu, M.; Zhu, S.H.; Liu, S.; Wang, M.; Qu, L. Identification and Thermal Stability of Purple-Fleshed Sweet Potato Anthocyanins in Aqueous Solutions with Various PH Values and Fruit Juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Lv, Y.; He, Q. Composition and Color Stability of Anthocyanin-Based Extract from Purple Sweet Potato. Food Sci. Technol. 2015, 35, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Ruiz-Aguirre, I.; Abadias, M.; Viñas, I.; Bobo, G.; Aguiló-Aguayo, I. Effect of Thermosonication on the Bioaccessibility of Antioxidant Compounds and the Microbiological, Physicochemical, and Nutritional Quality of an Anthocyanin-Enriched Tomato Juice. Food Bioprocess Technol. 2019, 12, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Álvarez, J.Á.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a Novel Method Based on Ultrasound-Assisted Extraction for the Quantification of Anthocyanins and Total Phenolic Compounds in Blueberry Samples (Vaccinium corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lin, C.; Chen, M.-H.; Chiang, P.-Y. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods 2019, 393, 393. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Han, Y.; Gu, Z.; Gu, F. Composition and Colour Stability of Anthocyanins Extracted from Fermented Purple Sweet Potato Culture. LWT Food Sci. Technol. 2008, 41, 1412–1416. [Google Scholar] [CrossRef]
- Li, J.; Walker, C.E.; Faubion, J.M. Acidulant and Oven Type Affect Total Anthocyanin Content of Blue Corn Cookies. J. Sci. Food Agric. 2011, 91, 38–43. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of Drying Methods on the Physical Properties and Microstructures of Mango (Philippine “Carabao” Var.) Powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Pua, C.K.; Hamid, N.S.A.; Tan, C.P.; Mirhosseini, H.; Rahman, R.B.A.; Rusul, G. Optimization of Drum Drying Processing Parameters for Production of Jackfruit (Artocarpus Heterophyllus) Powder Using Response Surface Methodology. LWT Food Sci. Technol. 2010, 43, 343–349. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of Spray-Drying, Drum-Drying and Freeze-Drying for β-Carotene Encapsulation and Preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Suriyajunhom, P.; Phongpipatpong, M. Optimization of Drum Drying Parameters for Gac Powder. MATEC Web Conf. 2018, 192, 192–195. [Google Scholar] [CrossRef]
- Chia, S.L.; Chong, G.H. Effect of Drum Drying on Physico-Chemical Characteristics of Dragon Fruit Peel (Hylocereus polyrhizus). Int. J. Food Eng. 2015, 11, 285–293. [Google Scholar] [CrossRef]
- Dao, V.T.T. Optimization of Drum Drying Process Parameters for Pumpkin Powder Production and Its Substitution in Rice Noodles. J. Sci. 2015, 3, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Kakade, R.H.; Das, H.; Ali, S. Performance Evaluation of a Double Drum Dryer for Potato Flake Production. J. Food Sci. Technol. 2011, 48, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Supprung, P.; Noomhorm, A. Optimization of Drum Drying Parameters for Low Amylose Rice (KDML105) Starch and Flour. Dry. Technol. 2003, 21, 1781–1795. [Google Scholar] [CrossRef]
- Valous, N.A.; Gavrielidou, M.A.; Karapantsios, T.D.; Kostoglou, M. Performance of a Double Drum Dryer for Producing Pregelatinized Maize Starches. J. Food Eng. 2002, 51, 171–183. [Google Scholar] [CrossRef]
- Tang, J.; Feng, H.; Shen, G.Q. Drum Drying. In Encyclopaedia of Agricultural, Food, and Biological Engineering; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 211–214. [Google Scholar]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Agricultural Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Chang, L.S.; Karim, R.; Abdulkarim, S.M.; Ghazali, H.M. Production and Characterization of Enzyme-Treated Spray-Dried Soursop (Annona muricata L.) Powder. J. Food Process. Eng. 2018, 41, 1–12. [Google Scholar] [CrossRef]
- Basilio-Atencio, J.; Condezo-Hoyos, L.; Repo-Carrasco-Valencia, R. Effect of Extrusion Cooking on the Physical-Chemical Properties of Whole Kiwicha (Amaranthus Caudatus L) Flour Variety Centenario: Process Optimization. LWT 2020, 128, 109426. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.F.; Zhao, Y.Y.; Mao, L.C. Effects of Drying Processes on the Antioxidant Properties in Sweet Potatoes. Agric. Sci. China 2010, 9, 1522–1529. [Google Scholar] [CrossRef]
- Azman, E.M.; House, A.; Charalampopoulos, D.; Chatzifragkou, A. Effect of Dehydration on Phenolic Compounds and Antioxidant Activity of Blackcurrant (Ribes nigrum L.) Pomace. Int. J. Food Sci. Technol. 2021, 56, 600–607. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Williams, P.; Pace, R.D. Identification of Anthocyanins in Muscadine Grapes with HPLC-ESI-MS. LWT Food Sci. Technol. 2009, 42, 819–824. [Google Scholar] [CrossRef]
- Azman, E.M.; Charalampopoulos, D.; Chatzifragkou, A. Acetic Acid Buffer as Extraction Medium for Free and Bound Phenolics from Dried Blackcurrant (Ribes nigrum L.) Skins. J. Food Sci. 2020, 85, 3745–3755. [Google Scholar] [CrossRef]
- Yea, C.S.; Addelia Nevara, G.; Muhammad, K.; Ghazali, H.M.; Karim, R. Physical Properties, Resistant Starch Content and Antioxidant Profile of Purple Sweet Potato Powder after 12 Months of Storage. Int. J. Food Prop. 2019, 22, 974–984. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Abdul Hamid, A. Optimization of Pulsed Ultrasound-Assisted Technique for Extraction of Phenolics from Pomegranate Peel of Malas Variety: Punicalagin and Hydroxybenzoic Acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Woodall, G.S.; Stewart, G.R. Do Anthocyanins Play a Role in UV Protection of the Red Juvenile Leaves of Syzygium? J. Exp. Bot. 1998, 49, 1447–1450. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.N.; Mat Noh, N.A.; Abdullah, E.N.; Yarmo, M.A.; Mat Piah, M.B.; Ku Bulat, K.H. Optimization of a Protease Extraction Using a Statistical Approach for the Production of an Alternative Meat Tenderizer from Spondias cytherea Roots. J. Food Process. Preserv. 2019, 43, e14192. [Google Scholar] [CrossRef]
- Shih, M.C.; Kuo, C.C.; Chiang, W. Effects of Drying and Extrusion on Colour, Chemical Composition, Antioxidant Activities and Mitogenic Response of Spleen Lymphocytes of Sweet Potatoes. Food Chem. 2009, 117, 114–121. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; De Wang, C.; Xiao, X.L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Xu, W.; Zheng, X. Analysis of the Anthocyanin Degradation in Blue Honeysuckle Berry under Microwave Assisted Foam-Mat Drying. Foods 2020, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Durge, A.V.; Sarkar, S.; Singhal, R.S. Stability of Anthocyanins as Pre-Extrusion Colouring of Rice Extrudates. Food Res. Int. 2013, 50, 641–646. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of Anthocyanins Isolated from Black Rice (Oryza sativa L.) and Their Degradation Kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Xu, E.; Pan, X.; Wu, Z.; Long, J.; Li, J.; Xu, X.; Jin, Z.; Jiao, A. Response Surface Methodology for Evaluation and Optimization of Process Parameter and Antioxidant Capacity of Rice Flour Modified by Enzymatic Extrusion. Food Chem. 2016, 212, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Ko, M.-J.; Chung, M.-S. Anthocyanin Structure and PH Dependent Extraction Characteristics from Blueberries (Vaccinium corymbosum) and Chokeberries (Aronia Melanocarpa) in Subcritical Water State. Foods 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of Two Anthocyanin Quantification Methods: HPLC and Spectrophotometric Methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Varela, P.; Poudroux, C.; Dessev, T.; Myhrer, K.; Rieder, A.; Zobel, H.; Sahlstrøm, S.; Knutsen, S.H. The Impact of Extrusion Parameters on Physicochemical, Nutritional and Sensorial Properties of Expanded Snacks from Pea and Oat Fractions. LWT 2019, 112, 108252. [Google Scholar] [CrossRef]








| Independent Variable | Units | Symbol | Coded and Uncoded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Citric acid (CA) | % | X1 | 0.5 | 1.0 | 1.5 |
| Steam pressure (SP) | kPa | X2 | 300 | 400 | 500 |
| Drum rotation speed (DS) | rpm | X3 | 1 | 2 | 3 |
| Run No. | Independent Variables | Response Variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA (%) | SP (kPa) | DS (rpm) | MC | aw | L* | a* | b* | Hue | Chroma | TAC | DPPH | FRAP | TPC | TFC | WSI | WAC | pH | |
| (%) | (mg/100 g) | (μmol TE/g) | (μmol TE/g) | (mg GAE/g) | (mg CE/g) | (%) | (%) | |||||||||||
| 1 | −1 | −1 | 0 | 6.57 | 0.387 | 52.27 | 37.33 | −0.39 | −0.60 | 37.34 | 107.743 | 23.120 | 49.287 | 13.539 | 4.478 | 27.42 | 704.28 | 4.24 |
| 2 | +1 | −1 | 0 | 7.45 | 0.391 | 50.10 | 44.47 | 5.58 | 7.15 | 44.82 | 124.089 | 25.175 | 52.229 | 14.336 | 4.683 | 29.03 | 693.50 | 3.50 |
| 3 | −1 | 1 | 0 | 5.34 | 0.332 | 51.42 | 37.99 | −0.93 | −1.40 | 38.00 | 104.939 | 25.916 | 50.957 | 13.588 | 4.707 | 27.71 | 706.83 | 4.24 |
| 4 | +1 | 1 | 0 | 5.83 | 0.290 | 51.27 | 44.44 | 5.03 | 6.46 | 44.72 | 117.145 | 22.670 | 52.682 | 12.808 | 4.587 | 28.38 | 695.01 | 3.50 |
| 5 | −1 | 0 | −1 | 4.80 | 0.306 | 50.11 | 36.62 | −0.45 | −0.71 | 36.62 | 101.825 | 24.934 | 49.038 | 14.336 | 4.719 | 27.74 | 702.49 | 4.25 |
| 6 | +1 | 0 | −1 | 6.26 | 0.316 | 48.95 | 44.11 | 5.84 | 7.54 | 44.49 | 116.382 | 23.747 | 49.762 | 13.588 | 4.465 | 29.79 | 665.47 | 3.50 |
| 7 | −1 | 0 | +1 | 6.07 | 0.321 | 51.61 | 38.23 | −1.39 | −2.09 | 38.26 | 105.661 | 28.973 | 61.998 | 14.635 | 4.265 | 28.98 | 734.52 | 4.25 |
| 8 | +1 | 0 | +1 | 7.54 | 0.366 | 50.21 | 44.67 | 5.54 | 7.07 | 45.01 | 116.625 | 30.787 | 60.855 | 14.252 | 4.410 | 30.29 | 752.05 | 3.51 |
| 9 | 0 | −1 | −1 | 8.31 | 0.417 | 48.31 | 42.00 | 4.08 | 5.55 | 42.20 | 115.997 | 20.506 | 46.529 | 12.400 | 4.142 | 28.94 | 664.68 | 3.78 |
| 10 | 0 | +1 | −1 | 7.26 | 0.374 | 48.20 | 41.52 | 4.34 | 5.97 | 41.75 | 110.971 | 20.405 | 41.500 | 11.800 | 4.052 | 29.07 | 663.25 | 3.78 |
| 11 | 0 | −1 | +1 | 9.97 | 0.473 | 47.31 | 43.33 | 4.61 | 6.08 | 43.57 | 116.194 | 23.371 | 47.519 | 13.048 | 4.011 | 29.83 | 698.76 | 3.78 |
| 12 | 0 | +1 | +1 | 9.25 | 0.462 | 48.57 | 42.82 | 4.52 | 6.02 | 43.05 | 117.236 | 25.496 | 51.326 | 12.180 | 4.050 | 30.93 | 727.66 | 3.78 |
| 13 a | 0 | 0 | 0 | 8.49 | 0.420 | 45.89 | 42.22 | 4.88 | 6.59 | 42.50 | 113.492 | 23.243 | 45.791 | 11.926 | 3.945 | 30.12 | 681.06 | 3.78 |
| 14 a | 0 | 0 | 0 | 8.21 | 0.418 | 45.26 | 42.15 | 5.04 | 6.82 | 42.45 | 114.199 | 24.622 | 44.029 | 12.200 | 4.230 | 29.76 | 685.32 | 3.78 |
| 15 a | 0 | 0 | 0 | 8.37 | 0.433 | 45.39 | 41.91 | 5.17 | 7.04 | 42.23 | 113.222 | 23.659 | 44.697 | 12.187 | 4.191 | 29.84 | 679.59 | 3.78 |
| Parameters | Fitted Models | R2 | p-Value (Regression) | p-Value (Lack of Fit) |
|---|---|---|---|---|
| MC | MC = −1.048 + 19.63 CA − 0.0057 SP + 0.776 DS − 9.278 CA × CA | 96.96 | 0.000 | 0.153 |
| aw | aw = 0.117 + 0.72 CA − 0.0003 SP + 0.0262 DS − 0.3579 CA × CA | 88.13 | 0.000 | 0.089 |
| L* | L = 93.350 − 32.72 CA − 0.1434 SP − 2.81 DS + 15.75 CA × CA + 0.0002 SP × SP + 0.770 DS × DS | 95.55 | 0.000 | 0.186 |
| a* | a = 29.195 + 17.24 CA − 0.0004 SP + 0.600 DS − 5.184 CA × CA | 99.01 | 0.000 | 0.185 |
| b* | b = −10.280 + 24.78 CA − 0.0011 SP − 0.068 DS − 9.247 CA × CA | 98.25 | 0.000 | 0.096 |
| Hue | Hu = −14.550 + 35.21 CA − 0.0014 SP − 0.160 DS − 13.48 CA × CA | 98.16 | 0.000 | 0.114 |
| Chroma | Ch = 28.801 + 18.24 CA − 0.0005 SP + 0.604 DS − 5.516 CA × CA | 99.13 | 0.000 | 0.156 |
| TAC | TAC = 133.500 + 33.47 CA − 0.2157 SP + 1.318 DS− 9.98 CA × CA + 0.0002 SP × SP | 94.46 | 0.000 | 0.065 |
| DPPH | DPPH = −17.430 − 9.26 CA + 0.2055 SP + 2.379 DS + 9.86 CA × CA − 0.0002 SP × SP − 0.0265 CA × SP | 91.87 | 0.000 | 0.312 |
| FRAP | FRAP = 76.910 − 60.2 CA + 0.0011 SP + 7.99 DS + 30.63 CA × CA + 3.09 DS × DS | 88.10 | 0.000 | 0.100 |
| TPC | TPC = 18.600 − 10.48 CA + 0.0042 SP − 1.585 DS + 6.677 CA × CA + 0.458 DS × DS − 0.0079 CA × SP | 97.05 | 0.000 | 0.317 |
| TFC | TFC = 6.017 − 3.61 CA + 0.0001 SP − 0.0806 DS + 1.802 CA × CA | 80.59 | 0.000 | 0.675 |
| WSI | WSI = 11.800 + 10.72 CA + 0.0548 SP + 0.561 DS − 4.657 CA × CA − 0.00006 SP × SP | 87.15 | 0.000 | 0.123 |
| WAC | WAC = 803.600 − 239.10 CA + 0.0394 SP − 41.9 DS + 87.0 CA × CA + 10.43 DS × DS + 27.27 CA × DS | 95.60 | 0.000 | 0.122 |
| pH | pH = 4.912 − 1.51 CA + 0.00001 SP − 0.0146 DS + 0.3846 CA × CA + 0.0036 DS × DS | 100.0 | 0.000 | 0.393 |
| Response | Regression Parameter Coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main Effects | Quadratic Effects | Interaction Effects | ||||||||
| x1 | x2 | x3 | x12 | x22 | x32 | x1 x2 | x1 x3 | x2 x3 | ||
| MC | p-value | 0.001 | 0.000 | 0.000 | 0.000 | - | - | - | - | - |
| Coef | 0.536 | −0.575 | 0.776 | −2.319 | - | - | - | - | - | |
| aw | p-value | 0.803 | 0.011 | 0.010 | 0.000 | - | - | - | - | - |
| Coef | 0.002 | −0.026 | 0.026 | −0.088 | - | - | - | - | - | |
| L* | p-value | 0.027 | 0.436 | 0.274 | 0.000 | 0.001 | 0.049 | - | - | - |
| Coef | −0.611 | 0.185 | 0.265 | 3.937 | 1.816 | 0.770 | - | - | - | |
| a* | p-value | 0.000 | 0.707 | 0.000 | 0.000 | - | - | - | - | - |
| Coef | 3.438 | −0.044 | 0.600 | −1.296 | - | - | - | - | - | |
| b* | p-value | 0.000 | 0.455 | 0.659 | 0.000 | - | - | - | - | - |
| Coef | 3.145 | −0.115 | −0.067 | −2.312 | - | - | - | - | - | |
| Hue | p-value | 0.000 | 0.506 | 0.453 | 0.000 | - | - | - | - | - |
| Coef | 4.128 | −0.141 | −0.160 | −3.369 | - | - | - | - | - | |
| Chroma | p-value | 0.000 | 0.671 | 0.000 | 0.000 | - | - | - | - | - |
| Coef | 3.603 | −0.049 | 0.604 | −1.379 | - | - | - | - | - | |
| Antho | p-value | 0.000 | 0.020 | 0.058 | 0.021 | 0.021 | - | - | - | - |
| Coef | 6.759 | −1.716 | 1.318 | −2.494 | 2.482 | - | - | - | - | |
| DPPH | p-value | 0.852 | 0.452 | 0.000 | 0.002 | 0.003 | - | 0.034 | - | - |
| Coef | −0.071 | 0.289 | 2.379 | 2.465 | −2.201 | - | −1.325 | - | - | |
| FRAP | p-value | 0.625 | 0.917 | 0.002 | 0.001 | - | - | - | - | - |
| Coef | 0.530 | 0.110 | 4.360 | 7.440 | - | - | - | - | - | |
| TPC | p-value | 0.116 | 0.002 | 0.014 | 0.000 | - | 0.004 | 0.008 | - | - |
| Coef | −0.139 | −0.368 | 0.249 | 1.669 | - | 0.458 | −0.394 | - | - | |
| TFC | p-value | 0.953 | 0.841 | 0.134 | 0.000 | - | - | - | - | - |
| Coef | −0.003 | 0.010 | −0.081 | 0.451 | - | - | - | - | - | |
| WSI | p-value | 0.002 | 0.523 | 0.007 | 0.001 | 0.020 | - | - | - | - |
| Coef | 0.704 | 0.108 | 0.561 | −1.164 | −0.672 | - | - | - | - | |
| WAC | p-value | 0.073 | 0.160 | 0.000 | 0.000 | - | 0.024 | - | 0.005 | - |
| Coef | −5.260 | 3.940 | 27.140 | 21.750 | - | 10.430 | - | 13.640 | - | |
| pH | p-value | 0.000 | 0.189 | 1.000 | 0.000 | - | 0.020 | - | - | - |
| Coef | −0.370 | 0.001 | 0.000 | 0.096 | - | 0.004 | - | - | - | |
| Process Parameters | Target | Experimental Range | Optimum Value | Desirability | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Citric acid (%) | range | 0.5 | 1.5 | 0.59 | |
| Steam pressure (kPa) | range | 300 | 500 | 499.8 | |
| Drum rotation speed (rpm) | range | 1 | 3 | 3 | |
| Responses | Predicted values | 0.8181 | |||
| MC (%) | minimize | 4.801 | 9.965 | 6.791 | |
| Ch | target | 36.619 | 45.009 | 39.487 | |
| a* | target | 36.617 | 44.667 | 39.190 | |
| TAC (mg/100 g) | target | 101.825 | 124.089 | 108.005 | |
| DPPH (µmol TE/g) | maximize | 20.405 | 30.787 | 27.530 | |
| FRAP (µmol TE/g) | maximize | 41.500 | 61.998 | 56.319 | |
| TPC (mg GAE/g) | maximize | 11.800 | 14.635 | 13.858 | |
| WAC (%) | maximize | 663.252 | 752.04 | 736.396 | |
| pH | maximize | 3.503 | 4.250 | 4.144 | |
| Peak | Retention Time (min) | m/z | Anthocyanin | ||
|---|---|---|---|---|---|
| MH+ | Aglycon | Other Fragment Ions | |||
| 1 | 11.63 | 773 | 287 | 611, 449 | Cyanidin 3-sophoroside-5-glucoside |
| 2 | 12.99 | 787 | 301 | 625, 463 | Peonidin 3-sophoroside-5-glucoside |
| 3 | 16.08 | 893 | 287 | 731, 449 | Cyanidin 3-p-hydroxybenzoylsophoroside-5-glucoside |
| 4 | 17.27 | 907 | 301 | 745, 463 | Peonidin 3-p-hydroxybenzoylsophoroside-5-glucoside |
| 5 | 18.24 | 949 | 287 | 787, 449 | Cyanidin 3-(6”-feruloylsophoroside)-5-glucoside |
| 6 | 19.38 | 963 | 301 | 801, 463 | Peonidin 3-(6”-feruloylsophoroside)-5-glucoside |
| 7 | 20.03 | 935 | 287 | 773, 449 | Cyanidin 3-(6”-caffeoyl sophoroside)-5-glucoside |
| 8 | 20.76 | 949 | 301 | 787, 463 | Peonidin 3-caffeoyl sophoroside-5-glucoside |
| 9 | 20.81 | 1097 | 287 | 935, 449 | Cyanidin 3-(6”,6” ‘-dicaffeoyl sophoroside)-5-glucoside |
| 10 | 21.50 | 1111 | 287 | 949, 449 | Cyanidin 3-(6”-caffeoyl-6” ‘-feruloylsophoroside)-5-glucoside |
| 11 | 21.96 | 1069 | 301 | 907, 463 | Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside |
| 12 | 22.52 | 1125 | 301 | 963, 463 | Peonidin 3-(6”-caffeoyl-6” ‘-feruloylsophoroside)-5-glucoside |
| Analysis Method | Anthocyanin Content (mg/100 g DM) |
|---|---|
| HPLC | |
| Cyanidin-based | 85.17 ± 1.64 |
| Peonidin-based | 65.91 ± 4.07 |
| Total anthocyanins | 151.08 ± 5.69 |
| pH differential method | |
| Total anthocyanins | 108.77 ± 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senevirathna, S.S.J.; Ramli, N.S.; Azman, E.M.; Juhari, N.H.; Karim, R. Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology. Foods 2021, 10, 1378. https://doi.org/10.3390/foods10061378
Senevirathna SSJ, Ramli NS, Azman EM, Juhari NH, Karim R. Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology. Foods. 2021; 10(6):1378. https://doi.org/10.3390/foods10061378
Chicago/Turabian StyleSenevirathna, Sri Sampath Janaka, Nurul Shazini Ramli, Ezzat Mohamad Azman, Nurul Hanisah Juhari, and Roselina Karim. 2021. "Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology" Foods 10, no. 6: 1378. https://doi.org/10.3390/foods10061378
APA StyleSenevirathna, S. S. J., Ramli, N. S., Azman, E. M., Juhari, N. H., & Karim, R. (2021). Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology. Foods, 10(6), 1378. https://doi.org/10.3390/foods10061378