Heavy Metals Presence in the Soil and Their Content in Selected Varieties of Chili Peppers in Slovakia
Abstract
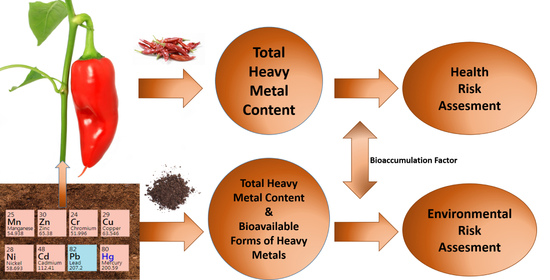
:1. Introduction
2. Materials and Methods
2.1. Study Location and Sample Collection
2.2. Chemical Analysis of the Soil
2.2.1. Determination of (pH/CaCl2)
2.2.2. Determination of Humus Content and Content of Organic Carbon (Cox) in Soil
2.2.3. Determination of Available Micronutrients (Ca, Mg, K, P) in Soil by Mehlich II
2.2.4. Determination of Total Heavy Metal Content in Soil
2.2.5. Determination of Mobile Forms of Heavy Metals in Soil
2.3. Chemical Analysis of the Plant Material
2.4. Environmental and Health Risk Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Agrochemical Characteristics, Contents of Nutrients and Heavy Metals in Soil
3.2. Concentration of Heavy Metals in Plant Samples
3.3. Statistical Analysis
3.3.1. Analysis of Variance
3.3.2. The Relationships between the Tested Parameters
3.3.3. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rai, K.P.; Sang, L.S.; Zhang, M.; Tsang, F.Y.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities of multi-metal contaminated soils: A case study in National Park of Alta Murgia (Apulia Region—Southern Italy). Int. J. Phytoremediat. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for in habitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and home grown vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Lu, S. Cd accumulation and transfer in pepper (Capsicum annuum L.) grown in typical soils of China: Pot experiments. Environ. Sci. Pollut. Res. 2019, 26, 36558–36567. [Google Scholar] [CrossRef]
- Paltseva, A.; Cheng, Z.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Mwilola, P.N.; Mukumbuta, I.; Shitumbanuma, V.; Chishala, B.H.; Uchida, Y.; Nakata, H.; Nakayama, S.; Ishizuka, N. Lead, zinc and cadmium accumulation, and associated health risks, in maize grown near the Kabwe Mine in Zambia in response to organic and inorganic soil amendments. Int. J. Environ. Res. Public Health 2020, 17, 9038. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Peer Review Workshop on Mercury Issues; Summary Report; Environmental Criteria and Assessment Office: Cincinnati, OH, USA, 1987.
- Agency for Toxic Substances and Disease Registry (ATSDR). Interaction Profile for Arsenic, Cadmium, Chromium and Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutics strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–331. [Google Scholar] [CrossRef]
- Caravanos, J.; Kevin, C.; Bret, E.; Landrigan, P.J.; Richard, F. The burden of disease from pediatric lead exposure at hazardous waste sites in 7 Asian countries. Environ. Res. 2013, 120, 119–125. [Google Scholar] [CrossRef]
- Jan, A.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Khan, H.; Jolly, Y.; Kabir, J.; Akter, S.; Salam, A. Assessing risk to human health for heavy metal contamination through street dust in the Southeast Asian Megacity: Dhaka, Bangladesh. Sci. Total Environ. 2019, 660, 1610–1622. [Google Scholar] [CrossRef]
- Etchevers, A.; Bretin, P.; Lecoffre, C.; Bidondo, M.L.; Le Strat, Y.; Glorennec, P.; Le Tertre, A. Blood lead levels and risk factors in young children in France, 2008–2009. Int. J. Hyg. Environ. Health 2014, 217, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Kastury, F.; Smith, E.; Doelsch, E.; Lombi, E.; Donnelley, M.; Cmielewski, P.; Parsons, D.; Scheckel, K.; Paterson, D.; de Jonge, M.; et al. In Vitro, in Vivo, and Spectroscopic Assessment of lead exposure reduction via ingestion and inhalation pathways using phosphate and iron amendments. Environ. Sci. Technol. 2019, 53, 10329–10341. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U.; Schoendorfer, N. Analysis of lead and cadmium in cereal products and duplicate diets of a small group of selected Brisbane children for estimation of daily metal exposure. J. Trace Elem. Med. Biol. 2018, 50, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.J.; Wang, F.; Ali, S.; Zhang, G. Toxic effects of cadmium on rice as affected by nitrogen fertilizer form. Plant. Soil 2005, 277, 359–365. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Moryani, H.T.; Kong, S.; Du, J.; Bao, J. Health Risk Assessment of Heavy Metals Accumulated on PM2.5 Fractioned Road Dust from Two Cities of Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 7124. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of total cancer and lung cancer with environmental exposure to cadmium: The meta-analytical evidence. Cancer Causes Control 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [Green Version]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef] [Green Version]
- Rawat, N.; Tiwari, V.K.; Singh, N.; Randhawa, G.S.; Singh, K.; Chhuneja, P.; Dhaliwal, H.S. Evaluation and utilization of Aegilops and wild Triticum species for enhancing iron and zinc content in wheat. Res. Crop. Evol. 2009, 56, 53–64. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, Ecology, In Situ and Ex Situ Conservation and Expected Actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Agnew, U.M.; Slesinger, T.L. Zinc Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.R.; Azcona-Cruz, M.I. Toxic effects of manganese. Rev. Espec. Médico Quirúrgicas 2018, 22, 71–75. [Google Scholar]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of chromium on the immune system. FEMS Microbiol. Immunol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001, 222, 149–158. [Google Scholar] [CrossRef]
- IARC. Chromium, nickel and welding. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1990; Volume 49, pp. 1–687. [Google Scholar]
- Stoj, C.; Kosman, D.J. Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: Implication for function. FEBS Lett. 2003, 554, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Kondo, T.; Komatsu, M.; Ohshiro, Y.; Li, C.; Kanehisa, N.; Kai, Y.; Fukuzumi, S. Functional Model of Dopamine.beta.-Hydroxylase. Quantitative Ligand Hydroxylation at the Benzylic Position of a Copper Complex by Dioxygen. J. Am. Chem. Soc. 1995, 117, 4714–4715. [Google Scholar] [CrossRef]
- Barber, R.G.; Grenier, Z.A.; Burkhead, J.L. Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease. Biomedicines 2021, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Myśliwa-Kurdziel, B.; Prasad, M.N.V.; Strzałtka, K. Photosynthesis in heavy metal stressed plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2004; pp. 146–181. [Google Scholar]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Frizera Vassallo, P.; Ronacher Simões, M.; Valentim Vassallo, D. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 949048. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Affum, G.A.O.; Osae, S.D.; Kwaansa-Ansah, E.E.; Miyittah, M.K. Quality assessment and potential health risk of heavy metals in leafy and non-leafy vegetables irrigated with groundwater and municipal-waste-dominated stream in the Western region, Ghana. Heliyon 2020, 6, e05829. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F. Distribution of seven heavy metals among hot pepper plant parts. J. Environ. Sci. Health Part B 2016, 51, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Antonious, G.F.; Kochhar, T.S. Mobility of Heavy Metals from Soil into Hot Pepper Fruits: A Field Study. Bull. Environ. Contam. Toxicol. 2008, 82, 59–63. [Google Scholar] [CrossRef]
- Aslam, R.; Ansari, M.Y.K.; Choudhary, S.; Bhat, T.M.; Jahan, N. Genotoxic effects of heavy metal cadmium on growth, biochemical, cyto-physiological parameters and detection of DNA polymorphism by RAPD in Capsicum annuum L.—An important spice crop of India. Saudi J. Biol. Sci. 2014, 21, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Aslam, R.; Bhat, T.M.; Choudhary, S.; Ansari, M.Y.K. An overview on genotoxicity of heavy metal in a spice crop (Capsicum annuum L.) in respect to cyto-morphological behavior. Caryologia 2017, 70, 42–47. [Google Scholar] [CrossRef]
- Dorne, J.L.; Kass, G.E.; Bordajandi, L.R.; Amzal, B.; Bertelsen, U.; Castoldi, A.F.; Heppner, C.; Eskola, M.; Fabiansson, S.; Ferrari, P.; et al. Human risk assessment of heavy metals: Principles and applications. Met. Ions Life Sci. 2011, 8, 27–60. [Google Scholar] [PubMed]
- Barlow, S.M.; Boobis, A.R.; Bridges, J.; Cockburn, A.; Dekant, W.; Hepburn, P.; Bánáti, D. The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. Technol. 2015, 46, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Relief Map of Slovakia. Available online: https://sk.wikipedia.org/wiki/S%C3%BAbor:Relief_Map_of_Slovakia.png (accessed on 28 May 2021).
- Background Map of the Nitra Region, Slovakia, Ready for the Geobox Template, Calibrated at en:Template:Geobox Locator Nitra Region, Outline Map of the Nitra Region, Slovakia, Ready for the Geobox Template, Calibrated at en:Template:Geobox Locator Nitra Region. Available online: https://sk.wikipedia.org/wiki/S%C3%BAbor:Nitra_Region_-_physical_map.png (accessed on 28 May 2021).
- Nikitin, B.A. Method for humus determination. AGROKHIMIIA 1999, 3, 91–93. [Google Scholar]
- Fiala, K. Obligatory methods of soil analysis. In Partial Monitoring System-Soil; VUPOP: Bratislava, Slovakia, 1999. (In Slovak) [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of the Mehlich 2 extractant. Commun. Soil Sci. Plant. Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Árvay, J.; Demková, L.; Hauptvogl, M.; Michalko, M.; Bajčan, D.; Stanovič, R.; Trebichalský, P. Assessment of environmental and health risks in former polymetallic ore mining and smelting area, Slovakia: Spatial distribution and accumulation of mercury in four different ecosystems. Ecotoxicol. Environ. Saf. 2015, 144, 236–244. [Google Scholar] [CrossRef]
- Jimoh, A.; Agbaji, E.B.; Ajibola, V.O.; Funtua, M.A. Application of Pollution Load Indices, Enrichment Factors, Contamination Factor and Health Risk Assessment of Heavy Metals Pollution of Soils of Welding Workshops at Old Panteka Market, Kaduna-Nigeria. Open J. Anal. Bioanal. Chem. 2020, 4, 11–19. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Linkeš, V.; Kobza, J.; Švec, M.; Ilka, P.; Pavlenda, P.; Barančíková, G.; Matúšková, L. Soil Monitoring of Slovak Republic Present State of Monitored Soil Properties; Vyskumny Ustav Podnej Urodnosti: Bratislava, Slovakia, 1997; pp. 39–51. [Google Scholar]
- Luo, W.; Lu, Y.; Gisey, J.P.; Wang, T.; Shi, Y.; Wang, G.; Xing, Y. Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir, China. Environ. Geochem. Health 2007, 29, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ahmed, K.; Al-Mamun, M.H.; Masunaga, S. Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Sci. Total Environ. 2015, 512–513, 94–102. [Google Scholar] [CrossRef]
- Yakun, S.; Xingmin, M.; Kairong, L.; Hongbo, S. Soil characterization and differential patterns of heavy metal accumulation in woody plants grown in coal gangue wastelands in Shaanxi, China. Environ. Sci. Pollut. Res. 2016, 23, 13489–13497. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Al-Mamun, M.H.; Hoque, M.F. Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ. Earth Sci. 2014, 73, 1837–1848. [Google Scholar] [CrossRef]
- Dryžalowska, A.; Falandysz, J. Bioconcentration of mercury by mushroom Xerocomus chrysenteron from the spatially distinct locations: Levels, possible intake and safety. Ecotoxicol. Environ. Saf. 2014, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.I.; Younes, M. Background to the ADI/TDI/PTWI. Regul Toxicol Pharm. 1999, 30, 109–113. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Mózsik, G. It remains a mystery why people living in hot climates consume spicier food. Temperature 2016, 3, 50–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addinsoft, XLSTAT. Analyse de Donneés et Statistique Avec MS Excel; Addinsoft: New York, NY, USA, 2014. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2005; Available online: http://www.rstudio.com/ (accessed on 1 May 2021).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Qiu, R. Factors Influencing Heavy Metal Availability and Risk Assessment of Soils at Typical Metal Mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Reid, B.J.; Li, G.; Zhu, Y.G. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environ. Int. 2014, 68, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Wang, X.; Tong, W.; Gurajala, H.K.; Lu, M.; Hamid, Y.; Feng, Y.; He, Z.; Yang, X. Distribution, availability and translocation of heavy metals in soil oilseed rape (Brassica napus L.) system related to soil properties. Environ. Pollut. 2019, 252, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yu, J.; Cao, Z.; Meng, M.; Yang, L.; Chen, Q. The Availability and Accumulation of Heavy Metals in Greenhouse Soils Associated with Intensive Fertilizer Application. Int. J. Environ. Res. Public Health 2020, 17, 5359. [Google Scholar] [CrossRef]
- Zejnulahu, B.; Kucaj, E.; Abazi, U.; Harizaj, F. Evaluation of heavy metal content in Capsicum annuum in Obiliq, Kosovo. Am. J. Eng. Res. 2017, 6, 186–189. [Google Scholar]
- Nenman, D.V.; Nanven, N.D.; Ezekiel, D.I. Trace metals accumulation in some irrigated vegetables grown around Heipang Village Plateau State. Glob. Eng. Technol. Rev. 2012, 2, 11–15. [Google Scholar]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clinic. Toxicol. S 2011, 3.2161, 0495. [Google Scholar] [CrossRef] [Green Version]
- USEPA. USEPA-Integrated Risk Information System (IRIS) on Manganese. In National Center for Environmental Assessment; Office of Research and Development: Washinton, DC, USA, 1999. [Google Scholar]
- Panda, S.K.; Patra, H.K. Does Cr(III) produces oxidative damage in excised wheat leaves. J. Plant. Biol. 2000, 27, 105–110. [Google Scholar]
- Panda, S.K.; Choudhury, S. Chromium stress in plants. Braz. J. Plant. Physiol. 2005, 17, 95–102. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health Part C 2016, 34, 1–32. [Google Scholar] [CrossRef]
- Wise, J.T.; Wang, L.; Xu, J.; Zhang, Z.; Shi, X. Oxidative stress of Cr (III) and carcinogenesis. In The Nutritional Biochemistry of Chromium (III); Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–340. [Google Scholar] [CrossRef]
- Gumi, A.M.; Sufiyanu, S. Effect of nickel on growth, pigmentation and ion homeostasis of Capsicum annum L. and Capsicum chinense L. Adv. Agric. Sci. Eng. Res. 2013, 3, 1430–1436, ISSN 2276-6723. [Google Scholar]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, Z.X.; Wang, D. Variations in Cd and Pb accumulations of hot pepper (Capsicum annuum L.) cultivars for screening pollution- and nitrate-safe cultivars. Pol. J. Environ. Stud. 2020, 29, 2597–2607. [Google Scholar] [CrossRef]
- Morikawa, C.K. Reducing Cadmium Accumulation in Fresh Pepper Fruits by Grafting. Hort. J. 2017, 86, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Lener, M.R.; Reszka, E.; Marciniak, W.; Lesicka, M.; Baszuk, P.; Jabłońska, E.; Lubiński, J. Blood cadmium levels as a marker for early lung cancer detection. J. Trace Elem. Med. Biol. 2021, 64, 126682. [Google Scholar] [CrossRef]
- Wilson, L.; Toxic Metals and Human Health. The Centre for Development. 2011. Available online: http://drlwilson.com/articles/TOXIC%20METALS.htm (accessed on 1 May 2021).
- Cambier, S.; Gonzalez, P.; Durrieu, G.; Bourdineaud, J.P. Cadmium-induced genotoxicity in zebrafish at environmentally relevant doses. Ecotox. Environ. Saf. 2010, 73, 312–319. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, X.; Li, T.; Hu, S.; Ji, J.; Wang, C. Characteristics of heavy metal transfer and their influencing factors in different soil–crop systems of the industrialization region, China. Ecotoxicol. Environ. Saf. 2016, 126, 193–201. [Google Scholar] [CrossRef]
- Árvay, J.; Tomáš, J.; Hauptvogl, M.; Massányi, P.; Harangozo, Ľ.; Tóth, T.; Stanovič, R.; Bryndzová, Š.; Bumbalová, M. Human exposure to heavy metals and possible public health risks via consumption of wild edible mushrooms from Slovak Paradise National Park, Slovakia. J. Environ. Sci. Health Part B 2015, 50, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Yea, X.; Zhang, Q.; Chen, D.; Hu, J.; Gao, N. Evaluation of cadmium transfer from soil to leafy vegetables: Influencing factors, transfer models, and indication of soil threshold contents. Ecotoxicol. Environ. Saf. 2018, 164, 355–362. [Google Scholar] [CrossRef]
- Reboredo, F. Zinc compartmentation in Halimione portulacoides (L.) Aellen and some effects on leaf ultrastructure. Environ. Sci. Pollut. Res. 2012, 19, 2644–2657. [Google Scholar] [CrossRef] [PubMed]





| Element | Wavelength (nm) | LoD (mg/L) | LoQ (mg/L) | Recovery (%) |
|---|---|---|---|---|
| Mn | 279.5 | 0.0250 | 0.0300 | 102.4 |
| Zn | 213.9 | 0.0870 | 0.1740 | 106.2 |
| Cu | 324.8 | 0.0876 | 0.0911 | 99.6 |
| Cr | 359.7 | 0.0300 | 0.050 | 92.2 |
| Ni | 232.0 | 0.3576 | 0.5646 | 104.9 |
| Pb | 217.0 | 0.0894 | 0.1411 | 93.7 |
| Cd | 228.8 | 0.0621 | 0.1200 | 91.6 |
| Hg | 253.7 | 0.02 * | 0.04 * | 97.1 |
| Ca | K | Mg | P | Nan | pH/CaCl2 | Humus (%) | COX (%) | |
|---|---|---|---|---|---|---|---|---|
| Min | 3879 | 139.15 | 305.68 | 168 | 43.9 | 7.47 | 3.06 | 1.77 |
| Max | 4150 | 147.12 | 338.15 | 180 | 46.34 | 7.63 | 3.17 | 1.83 |
| Median | 3975.5 | 143.065 | 316.46 | 173 | 45.08 | 7.55 | 3.085 | 1.8 |
| Average | 3995 | 143.1 | 319.19 | 174 | 45.1 | 7.55 | 3.1 | 1.8 |
| STDEV | 112.91 | 3.28 | 13.95 | 5.67 | 1.21 | 0.07 | 0.05 | 0.03 |
| Mn | Zn | Cr | Cu | Ni | Cd | Pb | Hg | |
|---|---|---|---|---|---|---|---|---|
| Minimum | 333.65 | 77.5 | 7.52 | 28.88 | 12.92 | 1.59 | 13.89 | 0.0125 |
| Maximum | 345.23 | 79.1 | 7.90 | 30.78 | 13.73 | 1.68 | 14.7 | 0.0132 |
| Median | 341.76 | 78.1 | 7.69 | 29.98 | 13.27 | 1645 | 14.29 | 0.0128 |
| Average | 340.6 | 78.2 | 7.7 | 29.9 | 13.3 | 1.64 | 14.30 | 0.013 |
| STDEV | 4.95 | 0.66 | 0.16 | 0.79 | 0.36 | 0.037 | 0.42 | 0.0003 |
| Contamination factor () | 0.85 | 1.10 | 0.22 | 1.19 | 0.67 | 16.4 | 0.72 | 0.16 |
| Potential ecological risk factor () | 0.85 | 1.10 | 0.44 | 5.98 | 3.33 | 492 | 3.57 | 6.41 |
| Geoaccumulation Index (Igeo) | −0.82 | −0.44 | −2.77 | −0.33 | −1.17 | 3.45 | −1.07 | −3.23 |
| Limit value | 150 | 60.0 | 50.0 | 0.70 | 70 | 0.50 | ||
| Threshold value | 100 | 40.00 | 30.0 | 0.50 | 50 |
| Mn | Zn | Cr | Cu | Ni | Cd | Pb | |
|---|---|---|---|---|---|---|---|
| Minimum | 0.14 | 0.18 | 0.03 | 0.19 | 0.14 | 0.1 | 0.24 |
| Maximum | 0.18 | 0.21 | 0.07 | 0.24 | 0.18 | 0.14 | 0.28 |
| Median | 0.16 | 0.185 | 0.05 | 0.225 | 0.16 | 0.12 | 0.26 |
| Average | 0.16 | 0.19 | 0.05 | 0.22 | 0.16 | 0.12 | 0.26 |
| STDEV | 0.018 | 0.014 | 0.018 | 0.022 | 0.018 | 0.018 | 0.018 |
| Critical value | 2.00 | 1.00 | 1.50 | 0.10 | 0.10 |
| mg/kg FW | Mn | Zn | Cr | Cu | Ni | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|
| C. annuum | ||||||||
| 1. Sigaretta di Bergamo | 1.32 ± 0.19 | 1.95 ± 0.25 | 0.23 ± 0.03 | 1.35 ± 0.15 | 0.23 ± 0.03 | 0.06 ± 0.003 | 0.36 ± 0.04 | 0.00003 ± 0.000005 |
| MINIMUM | 1.13 | 1.62 | 0.19 | 1.16 | 0.21 | 0.061 | 0.31 | 0.000025 |
| MAXIMUM | 1.54 | 2.23 | 0.25 | 1.52 | 0.27 | 0.068 | 0.41 | 0.000036 |
| MEDIAN | 1.31 | 1.98 | 0.24 | 1.35 | 0.22 | 0.065 | 0.36 | 0.000028 |
| BAF | 0.017 | 0.112 | 0.131 | 0.201 | 0.076 | 0.177 | 0.112 | 0.01 |
| 2. Cayenne Long Slim | 1.61 ± 0.19 | 1.39 ± 0.15 | 0.12 ± 0.02 | 0.82 ± 0.10 | 0.16 ± 0.02 | 0.02 ± 0.003 | 0.10 ± 0.02 | 0.00003 ± 0.00001 |
| MINIMUM | 1.42 | 1.22 | 0.10 | 0.70 | 0.14 | 0.012 | 0.08 | 0.000017 |
| MAXIMUM | 1.79 | 1.58 | 0.14 | 0.94 | 0.18 | 0.018 | 0.12 | 0.000042 |
| MEDIAN | 1.61 | 1.37 | 0.12 | 0.83 | 0.16 | 0.017 | 0.10 | 0.000028 |
| BAF | 0.024 | 0.088 | 0.079 | 0.138 | 0.06 | 0.049 | 0.036 | 0.011 |
| 3. Candlelight | 2.73 ± 0.34 | 2.74 ± 0.36 | 0.11 ± 0.02 | 1.75 ± 0.23 | 0.07 ± 0.01 | 0.04 ± 0.004 | 0.13 ± 0.02 | 0.00018 ± 0.00002 |
| MINIMUM | 2.27 | 2.30 | 0.09 | 1.48 | 0.05 | 0.035 | 0.11 | 0.000161 |
| MAXIMUM | 2.99 | 3.18 | 0.14 | 2.04 | 0.08 | 0.043 | 0.16 | 0.000207 |
| MEDIAN | 2.84 | 2.73 | 0.11 | 1.74 | 0.07 | 0.036 | 0.13 | 0.000184 |
| BAF | 0.026 | 0.082 | 0.103 | 0.125 | 0.03 | 0.116 | 0.056 | 0.042 |
| 4. Violet Cables | 2.24 ± 0.36 | 1.66 ± 0.20 | 0.19 ± 0.02 | 0.77 ± 0.07 | 0.10 ± 0.01 | 0.05 ± 0.007 | ND | 0.0001 ± 0.00003 |
| MINIMUM | 1.84 | 1.41 | 0.16 | 0.68 | 0.09 | 0.039 | ND | 0.000069 |
| MAXIMUM | 2.63 | 1.90 | 0.21 | 0.86 | 0.12 | 0.056 | ND | 0.000121 |
| MEDIAN | 2.24 | 1.66 | 0.19 | 0.77 | 0.10 | 0.047 | ND | 0.000111 |
| BAF | 0.033 | 0.105 | 0.121 | 0.128 | 0.039 | 0.140 | ND | 0.039 |
| C. chinense | ||||||||
| 5. Chupetinho | 2.34 ± 0.34 | 1.73 ± 0.23 | 0.20 ± 0.02 | 1.14 ± 0.12 | 0.08 ± 0.01 | 0.01 ± 0.002 | 0.04 ± 0.01 | 0.00045 ± 0.00013 |
| MINIMUM | 1.95 | 1.49 | 0.17 | 0.99 | 0.06 | 0.004 | 0.03 | 0.000269 |
| MAXIMUM | 2.70 | 2.04 | 0.22 | 1.28 | 0.09 | 0.008 | 0.05 | 0.000566 |
| MEDIAN | 2.35 | 1.69 | 0.20 | 1.14 | 0.08 | 0.006 | 0.04 | 0.000491 |
| BAF | 0.035 | 0.160 | 0.065 | 0.257 | 0.023 | 0.098 | 0.041 | 0.061 |
| 6. Scotch Bonnet Yellow | 2.64 ± 0.38 | 1.89 ± 0.11 | 0.23 ± 0.03 | 1.09 ± 0.11 | 0.12 ± 0.01 | 0.06 ± 0.006 | 0.23 ± 0.03 | 0.00016 ± 0.00006 |
| MINIMUM | 2.18 | 1.76 | 0.20 | 0.95 | 0.10 | 0.047 | 0.20 | 0.000073 |
| MAXIMUM | 3.10 | 2.03 | 0.26 | 1.22 | 0.13 | 0.060 | 0.27 | 0.000220 |
| MEDIAN | 2.64 | 1.89 | 0.23 | 1.10 | 0.12 | 0.058 | 0.23 | 0.000176 |
| BAF | 0.037 | 0.119 | 0.140 | 0.205 | 0.032 | 0.018 | 0.015 | 0.188 |
| 7. Bhut Jolokia Red | 2.01 ± 0.28 | 1.68 ± 0.21 | 0.18 ± 0.03 | 0.95 ± 0.12 | 0.15 ± 0.02 | 0.01 ± 0.002 | 0.15 ± 0.02 | 0.00003 ± 0.000004 |
| MINIMUM | 1.79 | 1.43 | 0.15 | 0.78 | 0.13 | 0.002 | 0.13 | 0.000020 |
| MAXIMUM | 2.41 | 1.93 | 0.22 | 1.05 | 0.17 | 0.008 | 0.17 | 0.000031 |
| MEDIAN | 1.93 | 1.68 | 0.17 | 0.98 | 0.15 | 0.005 | 0.15 | 0.000026 |
| BAF | 0.030 | 0.120 | 0.130 | 0.178 | 0.06 | 0.018 | 0.058 | 0.011 |
| 8. Bhut Jolokia White | 1.26 ± 0.22 | 1.12 ± 0.12 | 0.21 ± 0.02 | 0.70 ± 0.08 | 0.17 ± 0.02 | 0.08 ± 0.010 | 0.42 ± 0.05 | 0.00012 ± 0.00002 |
| MINIMUM | 1.06 | 0.98 | 0.19 | 0.60 | 0.15 | 0.072 | 0.38 | 0.000103 |
| MAXIMUM | 1.46 | 1.26 | 0.24 | 0.79 | 0.19 | 0.095 | 0.48 | 0.000141 |
| MEDIAN | 1.26 | 1.12 | 0.20 | 0.71 | 0.17 | 0.080 | 0.41 | 0.000122 |
| BAF | 0.020 | 0.076 | 0.143 | 0.125 | 0.068 | 0.262 | 0.086 | |
| Limit | 10.0 | 0.2 | 10.0 | 0.50 | 0.05 | 0.5 | 0.02 | |
| Maximal level | 0.05 | 0.10 | ||||||
| Tolerable intake | PMTDI 0.3–1 | PMTDI 0.5 | PTMI 0.025 | PTMI 0.025 | PTWI 0.004 | |||
| mg/kg DM | Mn | Zn | Cr | Cu | Ni | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|
| C. annuum | ||||||||
| 1. Sigaretta di Bergamo | 5.91 ± 0.85 a | 8.72 ± 1.11 a | 1.01 ± 0.12 a | 6.01 ± 0.65 a | 1.01 ± 0.13 a | 0.29 ± 0.01 a | 1.61 ± 0.19 a | 0.00013 ± 0.00002 a |
| MINIMUM | 5.06 | 7.25 | 0.83 | 5.20 | 0.92 | 0.27 | 1.39 | 0.00011 |
| MAXIMUM | 6.88 | 9.94 | 1.11 | 6.78 | 1.19 | 0.30 | 1.81 | 0.000159 |
| MEDIAN | 5.85 | 8.85 | 1.05 | 6.04 | 0.97 | 0.29 | 1.62 | 0.000124 |
| 2. Cayenne Long Slim | 8.01 ± 0.93 b | 6.90 ± 0.75 b | 0.61 ± 0.11 b | 4.10 ± 0.50 b | 0.80 ± 0.09 b | 0.08 ± 0.01 b | 0.51 ± 0.08 b | 0.00014 ± 0.00005 a |
| MINIMUM | 7.05 | 6.05 | 0.49 | 3.46 | 0.69 | 0.06 | 0.41 | 0.0001 |
| MAXIMUM | 8.93 | 7.87 | 0.71 | 4.69 | 0.90 | 0.09 | 0.61 | 0.000211 |
| MEDIAN | 8.02 | 6.84 | 0.62 | 4.13 | 0.80 | 0.09 | 0.51 | 0.000137 |
| 3. Candlelight | 9.00 ± 1.28 b | 6.45 ± 0.38 b | 0.79 ± 0.09 c | 3.73 ± 0.38 b | 0.40 ± 0.04 c | 0.19 ± 0.02 c | 0.80 ± 0.09 c | 0.00055 ± 0.00021 b |
| MINIMUM | 7.42 | 6.01 | 0.67 | 3.23 | 0.35 | 0.16 | 0.69 | 0.00025 |
| MAXIMUM | 10.56 | 6.90 | 0.90 | 4.15 | 0.45 | 0.21 | 0.91 | 0.00075 |
| MEDIAN | 9.01 | 6.45 | 0.79 | 3.76 | 0.40 | 0.20 | 0.80 | 0.0006 |
| 4. Violet Cables | 11.10 ± 1.77 c | 8.23 ± 0.98 a | 0.93 ± 0.11 a | 3.82 ± 0.37 b | 0.52 ± 0.05 d | 0.23 ± 0.04 d | ND | 0.00051 ± 0.00012 b |
| MINIMUM | 9.15 | 7.01 | 0.80 | 3.37 | 0.46 | 0.19 | ND | 0.00034 |
| MAXIMUM | 13.05 | 9.42 | 1.06 | 4.26 | 0.58 | 0.28 | ND | 0.000601 |
| MEDIAN | 11.10 | 8.24 | 0.94 | 3.83 | 0.52 | 0.23 | ND | 0.000551 |
| BAF | 0.033 | 0.105 | 0.121 | 0.128 | 0.039 | 0.140 | ND | 0.039 |
| C. chinense | ||||||||
| 5. Chupetinho | 12.01 ± 1.47 a | 12.01 ± 1.58 a | 0.50 ± 0.09 a | 7.69 ± 1.02 a | 0.30 ± 0.06 a | 0.17 ± 0.02 a | 0.59 ± 0.09 a | 0.00081 ± 0.00011 a |
| MINIMUM | 9.96 | 10.09 | 0.39 | 6.49 | 0.22 | 0.15 | 0.48 | 0.000709 |
| MAXIMUM | 13.13 | 13.95 | 0.60 | 8.98 | 0.35 | 0.19 | 0.70 | 0.00091 |
| MEDIAN | 12.47 | 12.01 | 0.50 | 7.64 | 0.32 | 0.16 | 0.59 | 0.000809 |
| 6. Scotch Bonnet Yellow | 12.60 ± 1.83 a | 9.32 ± 1.22 b | 1.08 ± 0.13 b | 6.13 ± 0.65 b | 0.42 ± 0.06 b | 0.03 ± 0.01 b | 0.21 ± 0.04 b | 0.00245 ± 0.00071 b |
| MINIMUM | 10.53 | 8.05 | 0.91 | 5.31 | 0.35 | 0.02 | 0.16 | 0.00145 |
| MAXIMUM | 14.53 | 10.99 | 1.19 | 6.89 | 0.49 | 0.04 | 0.25 | 0.003049 |
| MEDIAN | 12.67 | 9.13 | 1.11 | 6.15 | 0.42 | 0.03 | 0.21 | 0.002649 |
| 7. Bhut Jolokia Red | 11.30 ± 1.58 a | 9.41 ± 1.17 b | 1.00 ± 0.16 b | 5.33 ± 0.65 c | 0.83 ± 0.09 c | 0.03 ± 0.01 b | 0.83 ± 0.11 c | 0.00014 ± 0.00002 c |
| MINIMUM | 10.05 | 8.02 | 0.85 | 4.39 | 0.72 | 0.01 | 0.72 | 0.000114 |
| MAXIMUM | 13.50 | 10.81 | 1.21 | 5.90 | 0.94 | 0.04 | 0.98 | 0.000175 |
| MEDIAN | 10.83 | 9.41 | 0.97 | 5.51 | 0.83 | 0.03 | 0.82 | 0.000145 |
| 8. Bhut Jolokia White | 6.70 ± 1.17 b | 5.95 ± 0.63 c | 1.10 ± 0.12 b | 3.73 ± 0.43 d | 0.90 ± 0.10 c | 0.43 ± 0.0529 c | 2.23 ± 0.26 d | 0.00065 ± 0.00008 d |
| MINIMUM | 5.63 | 5.19 | 1.00 | 3.17 | 0.79 | 0.382 | 2.00 | 0.00055 |
| MAXIMUM | 7.74 | 6.70 | 1.27 | 4.21 | 1.02 | 0.506 | 2.54 | 0.00075 |
| MEDIAN | 6.72 | 5.95 | 1.07 | 3.77 | 0.90 | 0.4255 | 2.19 | 0.000651 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidiková, J.; Čeryová, N.; Šnirc, M.; Musilová, J.; Harangozo, Ľ.; Vollmannová, A.; Brindza, J.; Grygorieva, O. Heavy Metals Presence in the Soil and Their Content in Selected Varieties of Chili Peppers in Slovakia. Foods 2021, 10, 1738. https://doi.org/10.3390/foods10081738
Lidiková J, Čeryová N, Šnirc M, Musilová J, Harangozo Ľ, Vollmannová A, Brindza J, Grygorieva O. Heavy Metals Presence in the Soil and Their Content in Selected Varieties of Chili Peppers in Slovakia. Foods. 2021; 10(8):1738. https://doi.org/10.3390/foods10081738
Chicago/Turabian StyleLidiková, Judita, Natália Čeryová, Marek Šnirc, Janette Musilová, Ľuboš Harangozo, Alena Vollmannová, Jan Brindza, and Olga Grygorieva. 2021. "Heavy Metals Presence in the Soil and Their Content in Selected Varieties of Chili Peppers in Slovakia" Foods 10, no. 8: 1738. https://doi.org/10.3390/foods10081738
APA StyleLidiková, J., Čeryová, N., Šnirc, M., Musilová, J., Harangozo, Ľ., Vollmannová, A., Brindza, J., & Grygorieva, O. (2021). Heavy Metals Presence in the Soil and Their Content in Selected Varieties of Chili Peppers in Slovakia. Foods, 10(8), 1738. https://doi.org/10.3390/foods10081738