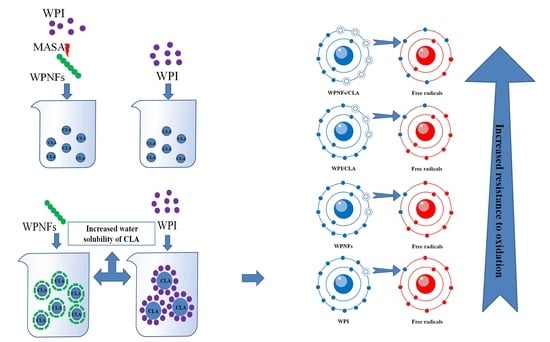
Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPNFs by MASA
2.3. Transmission Electron Microscopy (TEM)
2.4. Congo Red Spectroscopic Assay of WPNFs Formation
2.5. Determination of Surface Hydrophobicity
2.6. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.7. Pickering Emulsion Fabrication
2.8. Confocal Laser Scanning Microscope (CLSM)
2.9. Super Resolution Microscopes (SRM)
2.10. Cryogenic Scanning Electron Micrograph (Cryo-SEM)
2.11. Droplet Size Distribution and Zeta Potential
2.12. Emulsifying Activity and Emulsion Stability
2.13. Environmental Stress Stability
2.14. DPPH Radical Scavenging Activity Assay
2.15. ABTS+ Radical Scavenging Activity Assay
2.16. Statistical Analysis
3. Results and Discussion
3.1. TEM Micrograph of WPNFs Prepared by MASA
3.2. Congo Red Spectral Analysis
3.3. Surface Hydrophobicity
3.4. Building Blocks of WPNFs Prepared by Microwave Heating
3.5. Emulsion Micromorphology by CLSM
3.6. Emulsion Micromorphology by SRM
3.7. Emulsion Micromorphology by Cryo-SEM
3.8. Droplet Size and Zeta Potential
3.9. Emulsifying Properties
3.10. Influence of Ionic Strength and Temperature on Pickering Emulsion Stability
3.11. Antioxidant Activities of the Emulsion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Zhang, S.; Zhao, Z.; Liu, M.; Yin, X.; Zhou, Y.; Wu, Y.; Peng, Q. Highly effective lead (II) removal by sustainable alkaline activated β-lactoglobulin nanofibrils from whey protein. J. Clean. Prod. 2020, 255, 120297. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and Characterization of Coating Based on Protein Nanofibers and Polyphenol and Application for Salted Duck Egg Yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomadoni, B.; Capello, C.; Valencia, G.A.; Gutiérrez, T.J. Self-assembled proteins for food applications: A review. Trends Food Sci. Technol. 2020, 101, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, S.; Zhang, J.; Chi, Y.; Tian, B.; Li, L.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation of whey protein isolate nanofibrils by microwave heating and its application as carriers of lipophilic bioactive substances. LWT 2020, 125, 109213. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. In vitro digestion and stability under environmental stresses of ovotransferrin nanofibrils. Food Hydrocoll. 2020, 99, 105343. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Mohammadian, M.; Moghaddam, A.D.; Sharifan, A.; Dabaghi, P.; Hadi, S. Nanocomplexes of whey protein fibrillar aggregates and quercetin as novel multi-functional biopolymeric ingredients: Interaction, chemical structure, and bio-functionality. J. Iran. Chem. Soc. 2020, 17, 2481–2492. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroen, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Katsouli, M.; Tzia, C. Effect of lipid type, dispersed phase volume fraction and emulsifier on the physicochemical properties of nanoemulsions fortified with conjugated linoleic acid (CLA): Process optimization and stability assessment during storage conditions. J. Mol. Liq. 2019, 292, 13. [Google Scholar] [CrossRef]
- Fuke, G.; Nörnberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Chi, Y. Direct separation and purification of alpha-lactalbumin from cow milk whey by aqueous two-phase flotation of thermo-sensitive polymer/phosphate. J. Sci. Food Agric. 2021, 101, 4173–4182. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Farrokhi, F.; Ehsani, M.R.; Badii, F.; Hashemi, M. Structural and thermal properties of nanofibrillated whey protein isolate in the glassy state. LWT 2018, 95, 274–281. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int. J. Biol. Macromol. 2016, 89, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Doost, A.S.; Nasrabadi, M.N.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.; van der Linden, E. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Lee, W.; Choi, Y.; Lee, S.W.; Kim, I.; Lee, D.; Hong, Y.; Lee, G.; Yoon, D.S. Microwave-induced formation of oligomeric amyloid aggregates. Nanotechnology 2018, 29, 345604. [Google Scholar] [CrossRef]
- Zhu, Y.; Vanga, S.K.; Wang, J.; Raghavan, V. Effects of Ultrasonic and Microwave Processing on Avidin Assay and Secondary Structures of Egg White Protein. Food Bioprocess Technol. 2018, 11, 1974–1984. [Google Scholar] [CrossRef]
- Hettiarachchi, C.A.; Melton, L.D.; Gerrard, J.A.; Loveday, S. Formation of β-Lactoglobulin Nanofibrils by Microwave Heating Gives a Peptide Composition Different from Conventional Heating. Biomacromolecules 2012, 13, 2868–2880. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.S.; Lee, D.; Shin, D.; Shin, D.; Kim, J.; Kim, J. Microwave-Assisted Protein Digestion in a Plate Well for Facile Sampling and Rapid Digestion. Anal. Chem. 2017, 89, 10655–10660. [Google Scholar] [CrossRef] [PubMed]
- Hauser, N.J.; Basile, F. Online Microwave D-Cleavage LC-ESI-MS/MS of Intact Proteins: Site-Specific Cleavages at Aspartic Acid Residues and Disulfide Bonds. J. Proteome Res. 2008, 7, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Cardamone, M.; Puri, N.K. Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem. J. 1992, 282, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, W.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. Targeting Delivery System for Lactobacillus Plantarum Based on Functionalized Electrospun Nanofibers. Polymer 2020, 12, 1565. [Google Scholar] [CrossRef]
- Chen, X.-W.; Yang, X.-Q. Characterization of Orange Oil Powders and Oleogels Fabricated from Emulsion Templates Stabilized Solely by a Natural Triterpene Saponin. J. Agric. Food Chem. 2019, 67, 2637–2646. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Zhang, Y.; Li, X.; Liu, C.; Jiang, B.; Xu, J.; Sun, Z. Formation of whey protein isolate nanofibrils by endoproteinase GluC and their emulsifying properties. Food Hydrocoll. 2019, 94, 71–79. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and Characterization of a Microemulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, M.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z.; Li, J. Effective separation of prolyl endopeptidase from Aspergillus Niger by aqueous two phase system and its characterization and application. Int. J. Biol. Macromol. 2021, 169, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Bolder, S.G.; Sagis, L.; Venema, P.; Van Der Linden, E. Thioflavin T and Birefringence Assays to Determine the Conversion of Proteins into Fibrils. Langmuir 2007, 23, 4144–4147. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Adamcik, J.; Mezzenga, R. Amyloid Polymorphism in the Protein Folding and Aggregation Energy Landscape. Angew. Chem. Int. Ed. 2018, 57, 8370–8382. [Google Scholar] [CrossRef]
- Reynolds, N.P.; Adamcik, J.; Berryman, J.T.; Handschin, S.; Zanjani, A.A.H.; Li, W.; Liu, K.; Zhang, A.; Mezzenga, R. Author Correction: Competition between crystal and fibril formation in molecular mutations of amyloidogenic peptides. Nat. Commun. 2017, 8, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, A.; Gupta, T.P.; Bansal, S.; Kundu, B. Formation of amyloid fibrils by bovine carbonic anhydrase. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 930–935. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Emam-Djomeh, Z. Characterization of hydrogels formed by non-toxic chemical cross-linking of mixed nanofibrillated/heat-denatured whey proteins. J. Iran. Chem. Soc. 2019, 16, 2731–2741. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are Building Blocks of Heat-Induced Fibrillar Protein Aggregates of β-Lactoglobulin Formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Persson, K.H.; Blute, I.A.; Mira, I.C.; Gústafsson, J. Creation of well-defined particle stabilized oil-in-water nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 48–57. [Google Scholar] [CrossRef]
- Wiącek, A.E. Comparison of n-tetradecane/electrolyte emulsions properties stabilized by DPPC and DPPC vesicles in the electrolyte solution. Colloids Surf. B Biointerfaces 2011, 83, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jordan, A.; Nicolai, T.; Benyahia, L. Enhancement of the particle stabilization of water-in-water emulsions by modulating the phase preference of the particles. J. Colloid Interface Sci. 2018, 530, 505–510. [Google Scholar] [CrossRef]
- Subrot, P.S.; Irshaan, S.; Sivapratha, S.; Preetam, S. Nanoencapsulation strategies for lipid-soluble vitamins. Chem. Pap. 2018, 73, 1–16. [Google Scholar]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2019, 101, 105503. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa, C.; Armenta, E.G.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Wiącek, A.E. Electrokinetic properties of n-tetradecane/ethanol emulsions with DPPC and enzyme lipase or phospholipase A2. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 150–156. [Google Scholar] [CrossRef]
- Gonzalez-Jordan, A.; Benyahia, L.; Nicolai, T. Cold gelation of water in water emulsions stabilized by protein particles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 332–341. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Cheng, Y.; Zhu, J.; Huang, Q. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Nicolai, T.; Murray, B. Particle stabilized water in water emulsions. Food Hydrocoll. 2017, 68, 157–163. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Wiącek, A.E. Effect of ionic strength on electrokinetic properties of oil/water emulsions with dipalmitoylphosphatidylcholine. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 141–149. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Karaś, M.; Zięba, E.; Mężyńska, M.; Wiącek, A.E. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll. 2020, 101, 105539. [Google Scholar] [CrossRef]
- Stehl, D.; Hohl, L.; Schmidt, M.; Hübner, J.; Lehmann, M.; Kraume, M.; Schomaecker, R.; Von Klitzing, R. Characteristics of Stable Pickering Emulsions under Process Conditions. Chem. Ing. Tech. 2016, 88, 1806–1814. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Characterization of fibrillated antioxidant whey protein hydrolysate and comparison with fibrillated protein solution. Food Hydrocoll. 2016, 52, 221–230. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating based on Whey Protein Nanofibrils with TiO2 Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Łupina, K.; Biendl, M. Edible films based on gelatin, carboxymethyl cellulose, and their blends as carriers of potassium salts of iso-α-acids: Structural, physicochemical and antioxidant properties. Food Hydrocoll. 2021, 115, 106574. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. https://doi.org/10.3390/foods10081892
Jiao Q, Liu Z, Li B, Tian B, Zhang N, Liu C, Feng Z, Jiang B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods. 2021; 10(8):1892. https://doi.org/10.3390/foods10081892
Chicago/Turabian StyleJiao, Qiyang, Ziyuan Liu, Baoyun Li, Bo Tian, Ning Zhang, Chunhong Liu, Zhibiao Feng, and Bin Jiang. 2021. "Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly" Foods 10, no. 8: 1892. https://doi.org/10.3390/foods10081892
APA StyleJiao, Q., Liu, Z., Li, B., Tian, B., Zhang, N., Liu, C., Feng, Z., & Jiang, B. (2021). Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods, 10(8), 1892. https://doi.org/10.3390/foods10081892