Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solubility of Chrysin in Oil Phase
2.2. Development of Chrysin-Loaded Oil-in-Water Nanoemulsions
2.3. Quantification of Chrysin Encapsulated in Nanoemulsions
2.4. Determination of 2,2-Diphenyl-1-Picrylhydrazyl Scavenging Activity
2.5. Determination of Cholinesterase Inhibitory Activities
2.6. In Vitro Digestion and Bioaccessibility
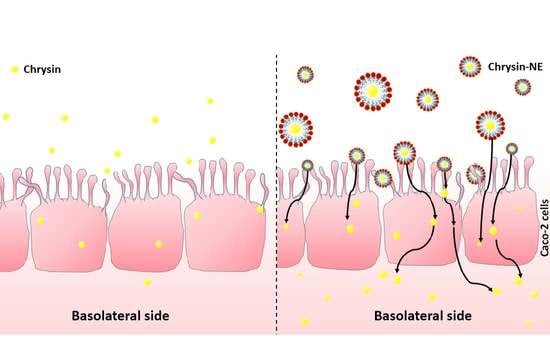
2.7. Cellular Uptake and Transport of Chrysin
2.8. Statistical Analysis
3. Results
3.1. Formulations and Characterizations of Chrysin Nanoemulsions (Chrysin-NE)
3.2. Effects of Nanoemulsion Preparation on Chrysin Biological Activities
3.3. Characteristics of Chrysin-NE during In Vitro Gastrointestinal Tract Digestion
3.4. Bioaccessibility of Chrysin and Chrysin-NE during In Vitro Gastrointestinal Tract Digestion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham Ul, H.; Yasmin, I.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Anand, K.V.; Mohamed Jaabir, M.S.; Thomas, P.A.; Geraldine, P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr. Gerontol. Int. 2012, 12, 741–750. [Google Scholar] [CrossRef]
- Tahir, M.; Sultana, S. Chrysin modulates ethanol metabolism in Wistar rats: A promising role against organ toxicities. Alcohol Alcohol. 2011, 46, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Mani, R.; Natesan, V.; Arumugam, R. Neuroprotective effect of chrysin on hyperammonemia mediated neuroinflammatory responses and altered expression of astrocytic protein in the hippocampus. Biomed. Pharmacother. 2017, 88, 762–769. [Google Scholar] [CrossRef]
- Dong, D.; Quan, E.; Yuan, X.; Xie, Q.; Li, Z.; Wu, B. Sodium Oleate-Based Nanoemulsion Enhances Oral Absorption of Chrysin through Inhibition of UGT-Mediated Metabolism. Mol. Pharm. 2017, 14, 2864–2874. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [PubMed]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef]
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica 1999, 29, 1241–1256. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Saupe, A.; Rades, T. Solid Lipid Nanoparticles. In Nanocarrier Technologies: Frontiers of Nanotherapy; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 41–50. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, W.; Liang, H.; Li, Y.; Liu, S.; Li, B. Chapter 1—Nanoemulsions for food: Properties, production, characterization, and applications. In Emulsions; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2016; pp. 1–36. [Google Scholar]
- Cardoso-Ugarte, G.A.; López-Malo, A.; Jiménez-Munguía, M.T. Chapter 7—Application of nanoemulsion technology for encapsulation and release of lipophilic bioactive compounds in food. In Emulsions; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2016; pp. 227–255. [Google Scholar]
- Zhang, Z.; McClements, D.J. Chapter 2—Overview of Nanoemulsion Properties: Stability, Rheology, and Appearance. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: London, UK, 2018; pp. 21–49. [Google Scholar]
- Vedagiri, A.; Sumathi, T. Enhanced blood–brain barrier transmigration using the novel chrysin embedded solid lipid nanoformulation: A salient approach on physico-chemical characterization, pharmacokinetics and biodistribution studies. Int. J. Pharm. Clin. Res. 2016, 8, 1574–1582. [Google Scholar]
- Komath, S.; Garg, A.; Wahajuddin, M. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles—Storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Rojsanga, P.; Bunsupa, S.; Brantner, A.H.; Sithisarn, P. Comparative Phytochemical Profiling and In Vitro Antioxidant Activity of Extracts from Raw Materials, Tissue-Cultured Plants, and Callus of Oroxylum indicum (L.) Vent. Evid. Based Complement. Alternat. Med. 2017, 2017, 6853212. [Google Scholar] [CrossRef]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Suttisansanee, U.; Kunkeaw, T.; Thatsanasuwan, N.; Tonglim, J.; Temviriyanukul, P. The investigation on cholinesterase and BACE1 inhibitory activities in various tea infusions. Walailak J. Sci. Tech. 2019, 16, 165–174. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Schwartz, S.J.; Failla, M.L. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and caco-2 human intestinal cells. J. Nutr. 2004, 134, 2280–2286. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.G.; Lumpkin, J.L.; Schwartz, S.J.; Failla, M. Digestive Stability, Micellarization, and Uptake of β-Carotene Isomers by Caco-2 Human Intestinal Cells. J. Agric. Food Chem. 2006, 54, 2780–2785. [Google Scholar] [CrossRef]
- Walsh, K.R.; Failla, M.L. Transport and metabolism of equol by Caco-2 human intestinal cells. J. Agric. Food Chem. 2009, 57, 8297–8302. [Google Scholar] [CrossRef]
- Punfa, W.; Suzuki, S.; Pitchakarn, P.; Yodkeeree, S.; Naiki, T.; Takahashi, S.; Limtrakul, P. Curcumin-loaded PLGA nanoparticles conjugated with anti- P-glycoprotein antibody to overcome multidrug resistance. Asian Pac. J. Cancer Prev. 2014, 15, 9249–9258. [Google Scholar] [CrossRef] [Green Version]
- Dimitrijevic, D.; Shaw, A.; Florence, A. Effects of Some Non-ionic Surfactants on Transepithelial Permeability in Caco-2 Cells. J. Pharm. Pharmacol. 2000, 52, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Spiegel, R.; Enz, A.; Veroff, A.E.; Cutler, N.R. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: Correlation with cognitive benefit. J. Neural Transm. 2002, 109, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimov, M.G.; Kudryavtsev, D.S.; Kryukova, E.V.; Fomina-Ageeva, E.V.; Zakharov, S.S.; Gretskaya, N.M.; Zinchenko, G.N.; Serkov, I.V.; Makhaeva, G.F.; Boltneva, N.P.; et al. Arachidonoylcholine and Other Unsaturated Long-Chain Acylcholines Are Endogenous Modulators of the Acetylcholine Signaling System. Biomolecules 2020, 10, 283. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Ji, Y.-S.; Lee, E.-S.; Hong, S.-T. Ostwald Ripening Stability of Curcumin-Loaded MCT Nanoemulsion: Influence of Various Emulsifiers. Prev. Nutr. Food Sci. 2016, 21, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; McClements, D.J. Chapter 1—Nanotechnology Approaches for Increasing Nutrient Bioavailability. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: London, UK, 2017; Volume 81, pp. 1–30. [Google Scholar]
- Chand Gupta, A.; Bawankule, D.U.; Verma, A.K.; Shanker, K. Nanoemulsion preconcentrate of a pentacyclic triterpene for improved oral efficacy: Formulation design and in-vivo antimalarial activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101734. [Google Scholar]
- Dehelean, C.A.; Feflea, S.; Ganta, S.; Amiji, M. Anti-angiogenic effects of betulinic acid administered in nanoemulsion formulation using chorioallantoic membrane assay. J. Biomed. Nanotechnol. 2011, 7, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xiang, C.; Wang, P.; Yin, Y.; Hou, Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int. J. Nanomed. 2017, 12, 2923–2931. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.H.; Nugroho, D.S.; Chang, J.Y.; Cheng, Y.S.; Liang, C.H.; Deng, M.J. Encapsulation and Characterization of Nanoemulsions Based on an Anti-oxidative Polymeric Amphiphile for Topical Apigenin Delivery. Polymers 2021, 13, 1016. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H.; McClements, D.J. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: Impact of physical form on quercetin bioaccessibility. Food Funct. 2013, 4, 162–174. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118 Pt A, 1211–1219. [Google Scholar] [CrossRef]
- Liao, Y.; Zhong, L.; Liu, L.; Xie, L.; Tang, H.; Zhang, L.; Li, X. Comparison of surfactants at solubilizing, forming and stabilizing nanoemulsion of hesperidin. J. Food Eng. 2020, 281, 110000. [Google Scholar] [CrossRef]
- Takenaka, M.; Ohkubo, T.; Okadome, H.; Sotome, I.; Itoh, T.; Isobe, S. Effective Extraction of Curcuminoids by Grinding Turmeric (Curcuma longa) with Medium-chain Triacylglycerols. J. Food Sci. Technol. 2013, 19, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Joung, H.J.; Choi, M.J.; Kim, J.T.; Park, S.H.; Park, H.J.; Shin, G.H. Development of Food-Grade Curcumin Nanoemulsion and its Potential Application to Food Beverage System: Antioxidant Property and In Vitro Digestion. J. Food Sci. 2016, 81, N745–N753. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Llinares, R.; Santos, J.; Trujillo-Cayado, L.A.; Ramírez, P.; Muñoz, J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT 2018, 97, 370–375. [Google Scholar] [CrossRef]
- Vedagiri, A.; Surekha, R.; Sumathi, T. Preparation, characterization and in-vitro cell viability assay of Chrysin loaded solid lipid nanoparticles as drug delivery system. Int. J. Pharm. Bio Sci. 2015, 6, P465–P478. [Google Scholar]
- Zhang, Y.; Zhao, J.; Afzal, O.; Kazmi, I.; Al-Abbasi, F.A.; Altamimi, A.S.A.; Yang, Z. Neuroprotective role of chrysin-loaded poly(lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22634. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Improving the In Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions. Foods 2020, 9, 447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancement of carotenoid bioaccessibility from carrots using excipient emulsions: Influence of particle size of digestible lipid droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef]
- Hunter, J.; Jepson, M.A.; Tsuruo, T.; Simmons, N.L.; Hirst, B.H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J. Biol. Chem. 1993, 268, 14991–14997. [Google Scholar] [CrossRef]
- Walle, U.K.; Galijatovic, A.; Walle, T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999, 58, 431–438. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, J.H.; Lee, Y.J. Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models. Asian J. Pharm. Sci. 2019, 14, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Jafari, S.M.; McClements, D.J. Chapter 19–Safety of Nanoemulsions and Their Regulatory Status. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: London, UK, 2018; pp. 613–616. [Google Scholar]




| Formulations | Oil Phase (% w/w) | Water Phase (% w/w) | |||
|---|---|---|---|---|---|
| MCT Oil | EtOH a | Span 80 | Tween 20 | Water | |
| Chrysin-NE 1 | 7.5 | 7.5 | 1 | 1 | 83 |
| Chrysin-NE 2 | 7.5 | 7.5 | 1.5 | 1.5 | 82 |
| Chrysin-NE 3 | 7.5 | 7.5 | 2 | 2 | 81 |
| Edible Oils | Chrysin Content (µg/g) |
|---|---|
| MCT | 1680.93 ± 54.15 |
| Rice bran | 178.09 ± 5.74 |
| Sunflower seed | 84.78 ± 2.73 |
| Grape seed | 41.30 ± 1.33 |
| Camellia seed | 40.90 ± 1.32 |
| Formulations | Size (nm) | PdI | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| Production Day | 5 Weeks | Production Day | 5 Weeks | Production Day | 5 Weeks | |
| Chrysin-NE 1 | 161 ± 1.96 | 173 ± 3.11 | 0.21 ± 0.01 | 0.27 ± 0.01 | −32 ± 0.61 | −30 ± 2.06 |
| Chrysin-NE 2 | 122 ± 1.50 | n/d | 0.23 ± 0.01 | n/d | −22 ± 0.51 | n/d |
| Chrysin-NE 3 | 110 ± 0.40 | n/d | 0.20 ± 0.02 | n/d | −23 ± 2.76 | n/d |
| Chrysin Nanoemulsions | Chrysin Content (µg/g) | Entrapment Efficiency (% w/w) |
|---|---|---|
| Production day | 174.21 ± 1.98 | 100.29 ± 0.53 |
| 1 week | 174.37 ± 0.91 | 99.19 ± 0.68 |
| 3 weeks | 171.28 ± 1.25 | 98.52 ± 0.72 |
| 5 weeks | 179.54 ± 0.71 | 101.75 ± 2.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, P.; Srinuanchai, W.; Suttisansanee, U.; Tuntipopipat, S.; Charoenkiatkul, S.; Praengam, K.; Chantong, B.; Temviriyanukul, P.; Nuchuchua, O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods 2021, 10, 1912. https://doi.org/10.3390/foods10081912
Ting P, Srinuanchai W, Suttisansanee U, Tuntipopipat S, Charoenkiatkul S, Praengam K, Chantong B, Temviriyanukul P, Nuchuchua O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods. 2021; 10(8):1912. https://doi.org/10.3390/foods10081912
Chicago/Turabian StyleTing, Pisamai, Wanwisa Srinuanchai, Uthaiwan Suttisansanee, Siriporn Tuntipopipat, Somsri Charoenkiatkul, Kemika Praengam, Boonrat Chantong, Piya Temviriyanukul, and Onanong Nuchuchua. 2021. "Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility" Foods 10, no. 8: 1912. https://doi.org/10.3390/foods10081912
APA StyleTing, P., Srinuanchai, W., Suttisansanee, U., Tuntipopipat, S., Charoenkiatkul, S., Praengam, K., Chantong, B., Temviriyanukul, P., & Nuchuchua, O. (2021). Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods, 10(8), 1912. https://doi.org/10.3390/foods10081912