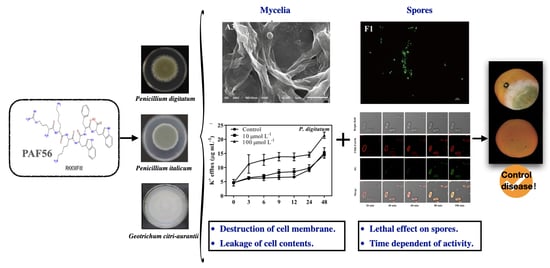
Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Peptide
2.2. Fungal Strains
2.3. Fruit and Treatment
2.4. Scanning Electron Microscope (SEM) Analysis
2.5. Measurement of Indicators Related to Change of Permeability of Cell Membrane
2.6. The Fungicidal Kinetics of PAF56 against Spores
2.7. Damage Effect of PAF56 on Membrane Permeability of Spores by Fluorescence Microscopy
3. Results
3.1. Analysis of PAF56’s Effect on the Induction of Defense Resistance in Citrus Fruit
3.2. Morphological Alterations of Fungal Mycelia in Response to PAF56
3.3. Effect of PAF56 on the Efflux of K+ and the Release of Cytoplasmic Constituents of Mycelia
3.4. Effect of PAF56 Treatment on the Membrane Permeability of Fungal Spores
3.5. Time-Kill Kinetics of PAF56 against Fungal Spores
3.6. Time-Lapse Confocal Fluorescence Microscopy Analyses of the Interaction of TMR-PAF56
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Futur. Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef]
- Keymanesh, K.; Soltani, S.; Sardari, S. Application of antimicrobial peptides in agriculture and food industry. World J. Microbiol. Biotechnol. 2009, 25, 933–944. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [Green Version]
- López-García, B.; Pérez-Payá, E.; Marcos, J.F. Identification of Novel Hexapeptides Bioactive against Phytopathogenic Fungi through Screening of a Synthetic Peptide Combinatorial Library. Appl. Environ. Microbiol. 2002, 68, 2453–2460. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.; Lopez-Garcia, B.; Marcos, J.F. Comparative Study of Antimicrobial Peptides to Control Citrus Postharvest Decay Caused byPenicillium digitatum. J. Agric. Food Chem. 2007, 55, 8170–8176. [Google Scholar] [CrossRef]
- López-García, B.; Veyrat, A.; Pérez-Payá, E.; González-Candelas, L.; Marcos, J.F. Comparison of the activity of antifungal hexapeptides and the fungicides thiabendazole and imazalil against postharvest fungal pathogens. Int. J. Food Microbiol. 2003, 89, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.; López-García, B.; Marcos, J.F. Studies on the Mode of Action of the Antifungal Hexapeptide PAF26. Antimicrob. Agents Chemother. 2006, 50, 3847–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-García, B.; Ubhayasekera, W.; Gallo, R.L.; Marcos, J.F. Parallel evaluation of antimicrobial peptides derived from the synthetic PAF26 and the human LL37. Biochem. Biophys. Res. Commun. 2007, 356, 107–113. [Google Scholar] [CrossRef]
- López-García, B.; Harries, E.; Carmona, L.; Campos-Soriano, L.; López, J.J.; Manzanares, P.; Gandía, M.; Coca, M.; Marcos, J.F. Concatemerization increases the inhibitory activity of short, cell-penetrating, cationic and tryptophan-rich antifungal peptides. Appl. Microbiol. Biotechnol. 2015, 99, 8011–8021. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Deng, L.; Yao, S.; Zeng, K. Control of green and blue mold and sour rot in citrus fruits by the cationic antimicrobial peptide PAF56. Postharvest Biol. Technol. 2018, 136, 132–138. [Google Scholar] [CrossRef]
- Jeong, R.-D.; Chu, E.-H.; Lee, G.W.; Cho, C.; Park, H.-J. Inhibitory effect of gamma irradiation and its application for control of postharvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 2016, 234, 1–8. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of Resistance to Penicillium digitatum in Grapefruit by the Yeast Biocontrol Agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Paul, S.; Dubey, R.; Maheshwari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2010, 22, 725–731. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Cheserek, M.J.; Su, G.; Le, G. Antibacterial activity and dual mechanisms of peptide analog derived from cell-penetrating peptide against Salmonella typhimurium and Streptococcus pyogenes. Appl. Microbiol. Biotechnol. 2012, 97, 1711–1723. [Google Scholar] [CrossRef]
- Puig, M.; Moragrega, C.; Ruz, L.; Calderón, C.E.; Cazorla, F.M.; Montesinos, E.; Llorente, I. Interaction of antifungal peptide BP15 with Stemphylium vesicarium, the causal agent of brown spot of pear. Fungal Biol. 2016, 120, 61–71. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Janda, T.; Pál, M.; Darkó, É.; Szalai, G. Use of Salicylic Acid and Related Compounds to Improve the Abiotic Stress Tolerance of Plants. Pract. Asp. 2017, 35–46. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Zhao, X.; She, X.; Du, Y.; Liang, X. Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pestic. Biochem. Physiol. 2007, 87, 78–84. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, X.; Du, Y. Oligochitosan: A plant diseases vaccine—A review. Carbohydr. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Pept. Sci. 2015, 55, 4–30. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Shi, Y.-H.; Tang, Y.-L.; Le, G.-W. The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptides 2009, 30, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J. Basic Microbiol. 2007, 47, 5–15. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [Green Version]
- Henriques, S.T.; Melo, M.N.; Castanho, M.A.R.B. Cell-penetrating peptides and antimicrobial peptides: How different are they? Biochem. J. 2006, 399, 1–7. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Feng, G.; Li, X.; Ruan, C.; Ming, J.; Zeng, K. Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage. Foods 2021, 10, 2031. https://doi.org/10.3390/foods10092031
Wang W, Feng G, Li X, Ruan C, Ming J, Zeng K. Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage. Foods. 2021; 10(9):2031. https://doi.org/10.3390/foods10092031
Chicago/Turabian StyleWang, Wenjun, Guirong Feng, Xindan Li, Changqing Ruan, Jian Ming, and Kaifang Zeng. 2021. "Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage" Foods 10, no. 9: 2031. https://doi.org/10.3390/foods10092031
APA StyleWang, W., Feng, G., Li, X., Ruan, C., Ming, J., & Zeng, K. (2021). Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage. Foods, 10(9), 2031. https://doi.org/10.3390/foods10092031