Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Relative Conductivity and Mass Loss
2.3. Sugar Content, Sweetness Index, and Taste Evaluation
2.4. Total Phenolics, Individual Phenolic Acid Content, and Antioxidant Capacity
2.5. Free Amino Acid Content Determination
2.6. RNA Isolation and Sequencing
2.7. Quantitative Real-Time PCR Validation
2.8. Statistical Analyses
3. Results
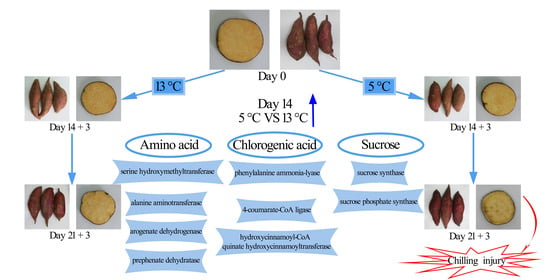
3.1. Chilling Injury, Relative Conductivity, and Mass Loss in Sweet Potato Tuberous Roots
3.2. Changes in Soluble Sugars, Sweetness Index, and Sensory Qualities
3.3. Changes in Total Phenolics, Free Phenolic Acid Content, and Antioxidant Capacity
3.4. Changes in Free Amino Acid Content in Sweet Potato Tuberous Roots during Storage
3.5. Sweet Potato Tuberous Roots Transcriptome Analysis at Different Storage Temperatures
3.5.1. Data Quality Evaluation and Analysis
3.5.2. Unigene Annotation Statistics
3.5.3. DEG Screening and Annotation Analysis
Comparison of Two DEG Groups
GO Database Annotation Analysis
KEGG Functional Annotation
3.5.4. Key Genes Involved in Sweet Potato Tuberous Roots Quality
3.6. Validation of RNA-Seq Results via qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2016, 15, 2751–2761. [Google Scholar] [CrossRef] [Green Version]
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Sheikha, A.; Ray, R.C. Potential impacts of bio-processing of sweet potato: Review. Crit. Rev. Food Sci. Nutr. 2015, 57, 455–471. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT, Crops and Livestock Products. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 1 July 2021).
- Masuda, D.; Fukuoka, N.; Goto, H.; Kano, Y. Effect of cold treatment after harvest on sugar contents and storability in sweet potato (Ipomoea batatas L.). Hortic. Res. 2007, 6, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Masuda, D.; Nishimura, K.; Ikeshita, Y. Relationship between invertase gene expression and sucrose concentration in the tuberous roots of sweet potato (Ipomoea batatas L. Lam.) during cold storage. J. Pomol. Hortic. Sci. 2014, 89, 229–235. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Ji, C.Y.; Chung, W.H.; Kim, H.S.; Jung, W.Y.; Kang, L.; Jeong, J.C.; Kwak, S.S. Transcriptome profiling of sweetpotato tuberous roots during low temperature storage. Plant Physiol. Biochem. 2017, 112, 97–108. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.Q.; Lu, G.Q. Low-temperature conditioning combined with cold storage inducing rapid sweetening of sweetpotato tuberous roots (Ipomoea batatas (L.) Lam) while inhibiting chilling injury. Postharvest Biol. Technol. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Liang, N.J.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Magwaza, L.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Keutgen, A.; Pawelzik, E. Modifications of taste-relevant compounds in strawberry fruit under NaCl salinity. Food Chem. 2007, 105, 1487–1494. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Ku, Y.H. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Apáti, P.; Szentmihályi, K.; Kristó, S.T.; Papp, I.; Vinkler, P.; Szoke, E.; Kéry, A. Herbal remedies of solidago—correlation of phytochemical characteristics and antioxidative properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.l. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2010, 89, 555–573. [Google Scholar] [CrossRef]
- Ru, L.; Chen, B.W.; Li, Y.X.; Wills, R.B.M.; Lv, Z.F.; Lu, G.Q.; Yang, H.Q. Role of sucrose phosphate synthase and vacuolar invertase in postharvest sweetening of immature sweetpotato tuberous roots (Ipomoea batatas (L.) Lam cv ‘Xinxiang’). Sci. Hortic. 2021, 282, 110007. [Google Scholar] [CrossRef]
- Walter, W.M.; Schadel, W.E. Distribution of phenols in “Jewel” sweet potato [Ipomoea batatas (L.) Lam.] roots. J. Agric. Food Chem. 1981, 29, 904–906. [Google Scholar] [CrossRef]
- Harrison, H.F.; Peterson, J.K.; Snook, M.E.; Bohac, J.R.; Jackson, D.M. Quantity and potential biological activity of caffeic acid in sweet potato [Ipomoea batatas (L.) Lam.] storage root periderm. J. Agric. Food Chem. 2003, 51, 2943–2948. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Effect of low temperature storage on phenolic composition and antioxidant activity of sweetpotatoes. Postharvest Biol. Technol. 2008, 47, 176–180. [Google Scholar] [CrossRef]
- Islam, S. Sweetpotato (Ipomoea batatas L.) Leaf: Its potential effect on human health and nutrition. J. Food Sci. 2006, 71, 13–21. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Methodology optimization for quantification of total phenolics and individual phenolic acids in sweetpotato (Ipomoea batatas L.) roots. J. Food Sci. 2010, 72, 412–416. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)-A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yahara, S.; Yoshimoto, M. Changes in polyphenolic content and radical-scavenging activity of sweet potato (Ipomoea batatas L.) during storage at optimal and low temperatures. J. Agric. Food Chem. 2007, 55, 10773–10778. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Moon, K.M.; Lee, B.; Cho, W.K.; Lee, B.S.; Kim, C.Y.; Ma, J.Y. Swertiajaponin as an anti-browning and antioxidant flavonoid. Food Chem. 2018, 252, 207. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Zhou, Z.L.; Li, H.M.; Yu, J.J.; Jiang, J.J.; Tang, Z.H.; Ma, D.F.; Zhang, B.H.; Han, Y.H.; Li, Z.Y. High throughput sequencing identifies chilling responsive genes in sweetpotato (Ipomoea batatas Lam.) during storage. Genomics 2019, 111, 1006–1017. [Google Scholar] [CrossRef]
- Lothar, E. Effect of metal ions on sucrose synthase from rice grains—A study on enzyme inhibition and enzyme topography. Glycobiology 1995, 5, 201–206. [Google Scholar] [CrossRef]
- Santoiani, C.S.; Tognetti, J.A.; Pontis, H.G.; Salerno, G.L. Sucrose and fructan metabolism in wheat roots at chilling temperatures. Physiol. Plant. 2010, 87, 84–88. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Uritani, I. Studies on chlorogenic acid biosynthesis in sweet potato root tissue using trans-cinnamic acid-2-14C and quinic acid-G-3H. Plant Cell Physiol. 1972, 13, 311–319. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Liu, S.; Ma, P.Y.; Jia, Z.D.; Xie, Y.Z.; Bian, X.F. Effects of exogenous phytohormones on chlorogenic acid accumulation and pathway-associated gene expressions in sweetpotato stem tips. Plant Physiol. Biochem. 2021, 164, 21–26. [Google Scholar] [CrossRef] [PubMed]







| DEG Set | DEG NUMBER | Upregulated | Downregulated |
|---|---|---|---|
| C0 vs. C14 | 1068 | 602 | 466 |
| C0 vs. CS14 | 9936 | 4850 | 5086 |
| Name | Gene ID | FPKM | Enzyme | ||
|---|---|---|---|---|---|
| C0 | C14 | CS14 | |||
| sucrose synthase | itf11g07860 | 88.50 | 67.61 | 912.29 | EC:2.4.1.13 |
| itf06g18950 | 4.20 | 7.05 | 113.25 | EC:2.4.1.13 | |
| itf02g07130 | 555.91 | 451.65 | 72.19 | EC:2.4.1.13 | |
| sucrose phosphate synthase | itf03g21140 | 84.66 | 96.66 | 19.06 | EC:2.4.1.14 |
| phenylalanine ammonia-lyase | itf09g14800 | 10.94 | 51.55 | 231.74 | EC:4.3.1.24 |
| itf09g14820 | 6.15 | 30.92 | 138.57 | EC:4.3.1.24 | |
| itf15g00190 | 1.69 | 24.46 | 56.15 | EC:4.3.1.24 | |
| 4-coumarate-CoA ligase | itf11g10280 | 61.32 | 70.53 | 166.77 | EC:6.2.1.12 |
| hydroxycinnamoyl-CoA quinate hydroxycinnamoy- ltransferase | itf07g23450 | 100.19 | 220.02 | 448.15 | EC:2.3.1.133 |
| serine hydroxymethyltransferase | itf09g04020 | 26.86 | 32.19 | 60.77 | EC:2.1.2.1 |
| alanine aminotransferase | itf11g08270 | 44.75 | 47.50 | 283.38 | EC:2.6.1.2 |
| arogenate dehydrogenase | itf10g08090 | 3.86 | 5.57 | 33.58 | EC:1.3.1.78 |
| itf12g23730 | 1.77 | 2.48 | 19.53 | EC:1.3.1.78 | |
| arogenate dehydratase/ prephenate dehydratase | itf07g12100 | 0.16 | 3.86 | 12.61 | EC:4.2.1.91 4.2.1.51 |
| itf14g14710 | 11.57 | 14.87 | 27.00 | EC:4.2.1.91 4.2.1.51 | |
| tyrosine aminotransferase | itf02g15070 | 38.52 | 20.45 | 7.71 | EC:2.6.1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Chen, L.; Chen, G.; Li, Y.; Yang, H. Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study. Foods 2021, 10, 2079. https://doi.org/10.3390/foods10092079
Zhou S, Chen L, Chen G, Li Y, Yang H. Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study. Foods. 2021; 10(9):2079. https://doi.org/10.3390/foods10092079
Chicago/Turabian StyleZhou, Shuqian, Lu Chen, Gang Chen, Yongxin Li, and Huqing Yang. 2021. "Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study" Foods 10, no. 9: 2079. https://doi.org/10.3390/foods10092079
APA StyleZhou, S., Chen, L., Chen, G., Li, Y., & Yang, H. (2021). Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study. Foods, 10(9), 2079. https://doi.org/10.3390/foods10092079