Banana Pseudo-Stem Increases the Water-Holding Capacity of Minced Pork Batter and the Oxidative Stability of Pork Patties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients
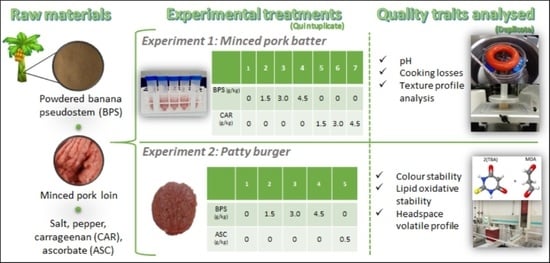
2.2. Experimental Plan
2.3. Experiment 1: Preparation of Lean-Pork Patty Batter
2.4. Experiment 1: Evaluation of Water-Holding Capacity and Texture of Cooked Pork Batter
2.5. Experiment 2: Preparation of Lean-Pork Patties
2.6. Experiment 2: Evaluation of the Colour and Lipid Oxidative Stability of Patties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1: Effect of BPS on the Water-Holding Capacity and Texture of Pork Batter
3.2. Experiment 2: The Effect of BPS on Colour and Lipid Oxidative Stability of Burger Patties
3.3. Experiment 2: Headspace Volatile Composition in Cooked Patties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekhit, A.E.D.; Geesink, G.H.; Ilian, M.A.; Morton, J.D.; Bickerstaffe, R. The effects of natural antioxidants on oxidative processes and metmyoglobin reducing activity in beef patties. Food Chem. 2003, 81, 175–187. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Shahidi, F. Oxidative stability and shelf life of meat and meat products. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; Academic Press: New York, NY, USA, 2016; pp. 373–389. ISBN 9781630670566. [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Fava, F.; Zanaroli, G.; Vannini, L.; Guerzoni, E.; Bordoni, A.; Viaggi, D.; Robertson, J.; Waldron, K.; Bald, C.; Esturo, A.; et al. New advances in the integrated management of food processing by-products in Europe: Sustainable exploitation of fruit and cereal processing by-products with the production of new food products (NAMASTE EU). New Biotechnol. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, N.; Belgacem, M.N.; Torres, I.C.; Moura, J.C.V.P. Chemical composition and pulping of banana pseudo-stems. Ind. Crops Prod. 2004, 19, 147–154. [Google Scholar] [CrossRef]
- Li, K.; Fu, S.; Zhan, H.; Zhan, Y.; Lucia, L.A. Analysis of the chemical composition and morphological structure of banana pseudo-stem. BioResources 2010, 5, 576–585. [Google Scholar]
- Mohapatra, D.; Mishra, S.; Sutar, N. Banana and its by-product utilisation: An overview. Sci. Ind. Res. 2010, 69, 323–329. [Google Scholar]
- Bhaskar, J.J.; Mahadevamma, S.; Chilkunda, N.D.; Salimath, P.V. Banana (Musa sp. var. elakki bale) flower and pseudostem: Dietary fiber and associated antioxidant capacity. J. Agric. Food Chem. 2012, 60, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.A.; Ho, L.H.; Azahari, B.; Bhat, R.; Cheng, L.H.; Ibrahim, M.N.M. Chemical and functional properties of the native banana (Musa acuminata × balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours. Food Chem. 2011, 128, 748–753. [Google Scholar] [CrossRef]
- Saravanan, K.; Aradhya, S.M. Polyphenols of pseudostem of different banana cultivars and their antioxidant activities. J. Agric. Food Chem. 2011, 59, 3613–3623. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.H.; Abdul Aziz, N.A.; Azahari, B. Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana (Musa acuminata × balbisiana cv. Awak) pseudo-stem flour. Food Chem. 2013, 139, 532–539. [Google Scholar] [CrossRef]
- Anusuya, N.; Gomathi, R.; Tharani, J.; Murugesan, G.S. Impact of polyphenols from banana pseudostem on sunflower oil stability. Food Sci. Biotechnol. 2013, 22, 773–780. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official’s Analytical Chemists, 17th ed.; AOAC: Arlington, TX, USA, 2003. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Osorio, M.T.; Zumalacárregui, J.M.; Bermejo, B.; Lozano, A.; Figueira, A.C.; Mateo, J. Effect of ewe’s milk versus milk-replacer rearing on mineral composition of suckling lamb meat and liver. Small Rumin. Res. 2007, 68, 296–302. [Google Scholar] [CrossRef]
- Feiner, G. Fresh sausages. In Meat Products Handbook: Practical Science and Technology; Woodhead Publishing: Cambridge, UK, 2006; pp. 297–309. [Google Scholar]
- Trius, A.; Sebranek, J.G. Carrageenans and their use in meat products. Crit. Rev. Food Sci. Nutr. 1996, 36, 69–85. [Google Scholar] [CrossRef]
- Lin, K.-W.; Mei, M.-Y. Influences of gums, soy protein isolate, and heating temperatures on reduced-fat meat batters in a model system. J. Food Sci. 2000, 65, 48–52. [Google Scholar] [CrossRef]
- Townsend, W.; Witnauer, L.; Riloff, J.; Swift, C. Comminuted meat emulsions: Differential thermal analysis of fat transition. Food Technol. 1968, 22, 319. [Google Scholar]
- Nam, K.C.; Ahn, D.U. Use of antioxidants to reduce lipid oxidation and off-odor volatiles of irradiated pork homogenates and patties. Meat Sci. 2003, 63, 1–8. [Google Scholar] [CrossRef]
- Carballo, D.E.; Caro, I.; Andrés, S.; Giráldez, F.J.; Mateo, J. Assessment of the antioxidant effect of astaxanthin in fresh, frozen and cooked lamb patties. Food Res. Int. 2018, 111, 342–350. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; Da Silva, J.K.R.; Setzer, W.N. Volatiles of black pepper fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondjoyan, N.; Berdagué, J.-L. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds; Laboratoire Flaveur, Institut National de la Reserche Agronomique: Theix, France, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020; p. 20899. Available online: https://webbook.nist.gov/chemistry/ (accessed on 10 January 2021).
- Ayadi, M.A.; Kechaou, A.; Makni, I.; Attia, H. Influence of carrageenan addition on turkey meat sausages properties. J. Food Eng. 2009, 93, 278–283. [Google Scholar] [CrossRef]
- Pietrasik, Z. Binding and textural properties of beef gels processed with κ-carrageenan, egg albumin and microbial transglutaminase. Meat Sci. 2003, 63, 317–324. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, R.; Pedreschi, F.; Valencia, E.; Díaz, O.; Bastías, J.; Muñoz, O. Kinetic modelling of deterioration of frozen industrial burgers based on oxidative rancidity and color. J. Food Process. Preserv. 2018, 42, e13655. [Google Scholar] [CrossRef]
- Akarpat, A.; Turhan, S.; Ustun, N.S. Effects of hot-water extracts from myrtle, rosemary, nettle and lemon balm leaves on lipid oxidation and color of beef patties during frozen storage. J. Food Process. Preserv. 2008, 32, 117–132. [Google Scholar] [CrossRef]
- AMSA. Guidelines for Meat Color Evaluation; American Meat Science Association: Champaign, IL, USA, 2012; Available online: https://meatscience.org/docs/default-source/publications-resources/hot-topics/2012_12_meat_clr_guide.pdf?sfvrsn=d818b8b3_0 (accessed on 31 August 2021).
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Resconi, V.; Escudero, A.; Campo, M. The Development of Aromas in Ruminant Meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Oh, W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: An overview. J. Food Bioact. 2020, 10. [Google Scholar] [CrossRef]
- Wang, R.; Huang, F.; Zhang, L.; Liu, Q.; Zhang, C.; Zhang, H. Changes in the texture, microstructures, colour and volatile compounds of pork meat loins during superheated steam cooking. Int. J. Food Sci. Technol. 2019, 54, 2821–2830. [Google Scholar] [CrossRef]
- Kim, H.W.; Miller, D.K.; Lee, Y.J.; Kim, Y.H.B. Effects of soy hull pectin and insoluble fiber on physicochemical and oxidative characteristics of fresh and frozen/thawed beef patties. Meat Sci. 2016, 117, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Effect of pork meat proteins on the binding of volatile compounds. Food Chem. 2008, 108, 1226–1233. [Google Scholar] [CrossRef]
- Shahidi, F. Lipid-derived flavors in meat products. In Meat Processing: Improving Quality; Kerry, J.P., Kerry, J.F., Ledward, D.A., Eds.; Woodhead Publishing: Cambridge, UK, 2002; pp. 105–122. [Google Scholar]
- Aaslyng, M.D.; Meinert, L. Meat flavour in pork and beef—From animal to meal. Meat Sci. 2017, 132, 112–117. [Google Scholar] [CrossRef]
| Component | |
|---|---|
| Moisture (%) 1 | 10.3 |
| Crude protein (%) 1 | 6.8 |
| Ether extract (%) 1 | 0.90 |
| Ashes (%) 1 | 28.8 |
| Fibre | |
| Total dietary fibre (%) 1 | 46.5 |
| Neutral detergent fibre (%) 2 | 39.2 |
| Acid detergent fibre (%) 2 | 27.4 |
| Total extractable polyphenols (%) 3 | 2.32 |
| Mineral content 4 | |
| Potassium (%) | 22.1 |
| Phosphorous (%) | 0.78 |
| Calcium (%) | 0.40 |
| Magnesium (%) | 0.31 |
| Sodium (%) | 0.11 |
| Iron (mg/100 g) | 37.5 |
| Manganese (mg/100 g) | 3.76 |
| Zinc (mg/100 g) | 3.20 |
| Copper (mg/100 g) | 0.95 |
| L: 1.5 g/kg | M: 3.0 g/kg | H: 4.5 g/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | BPS | CAR | BPS | CAR | BPS | CAR | SEM | p-Value | |
| pH | 5.68 d | 5.70 c | 5.65 e | 5.73 b | 5.64 e | 5.76 a | 5.65 e | 0.011 | *** |
| Cooking loss | 24.7 a | 22.6 ab | 21.8 bc | 19.0 c | 17.8 cd | 17.6 cd | 16.0 d | 1.26 | *** |
| Solids | 2.66 | 2.43 | 2.62 | 2.33 | 2.34 | 2.11 | 2.21 | 0.200 | NS |
| Hardness (N) | 234 a | 220 ab | 213 bc | 219 bc | 218 bc | 205 c | 218 bc | 7.29 | * |
| Elasticity | 0.429 | 0.416 | 0.415 | 0.415 | 0.417 | 0.419 | 0.433 | 0.010 | NS |
| Cohesiveness | 0.736 | 0.786 | 0.788 | 0.785 | 0.741 | 0.764 | 0.753 | 0.027 | NS |
| Chewiness (N) | 73.9 | 72.6 | 69.1 | 72.0 | 67.2 | 65.8 | 70.8 | 4.45 | NS |
| CON | ASC | LBPS | MBPS | HBPS | SEM | p-Value | |
|---|---|---|---|---|---|---|---|
| Colour values | |||||||
| L* | |||||||
| Fresh | 47.8 a | 47.9 a | 45.9 b | 45.0 c | 44.1 d | 0.224 | *** |
| Frozen stored | 45.8 | 45.9 | 44.5 | 43.5 | 42.3 | 1.073 | NS |
| a* | |||||||
| Fresh | 4.59 1 | 4.64 1 | 4.81 1 | 4.89 1 | 5.12 1 | 0.181 | NS |
| Frozen stored | 2.74 2 | 3.13 2 | 3.28 2 | 3.56 2 | 3.93 2 | 0.292 | NS |
| b* | |||||||
| Fresh | 11.8 | 11.8 | 12.1 | 12.2 | 12.4 | 0.290 | NS |
| Frozen stored | 10.3 | 10.4 | 11.0 | 11.3 | 11.6 | 0.383 | NS |
| Chroma | |||||||
| Fresh | 12.7 1 | 12.7 1 | 13.0 1 | 13.1 1 | 13.5 1 | 0.310 | NS |
| Frozen stored | 10.6 2 | 10.9 2 | 11.5 2 | 11.9 2 | 12.2 2 | 0.389 | NS |
| Hue angle | |||||||
| Fresh | 69.1 2 | 68.7 2 | 68.4 2 | 68.1 2 | 67.6 2 | 0.715 | NS |
| Frozen stored | 75.1 1 | 73.5 1 | 73.3 1 | 72.3 1 | 71.2 1 | 1.037 | NS |
| Reflectance ratios & | |||||||
| Rλ630/Rλ580 | |||||||
| Fresh | 1.88 a1 | 1.91 a1 | 1.70 b1 | 1.59 bc1 | 1.55 c1 | 0.017 | *** |
| Frozen stored | 1.35 2 | 1.43 2 | 1.34 2 | 1.33 2 | 1.32 2 | 0.027 | NS |
| Decrease $ | 0.529 a | 0.477 a | 0.368 ab | 0.262 b | 0.223 b | 0.017 | ** |
| Rλ572/Rλ525 | |||||||
| Fresh | 0.875 2 | 0.869 2 | 0.936 2 | 0.985 2 | 1.015 | 0.002 | *** |
| Frozen stored | 0.998 1 | 0.975 1 | 1.029 1 | 1.048 1 | 1.065 | 0.010 | NS |
| Increase $ | 0.123 a | 0.105 a | 0.092 ab | 0.063 bc | 0.050 c | 0.002 | *** |
| CON | ASC | LBPS | MBPS | HBPS | SEM | p-Value | |
|---|---|---|---|---|---|---|---|
| Not stored | 0.60 a3 | 0.14 e3 | 0.44 b3 | 0.32 c3 | 0.24 d3 | 0.022 | *** |
| Refrigerated stored & | 2.49 a1 | 1.23 c1 | 1.99 b1 | 1.27 c1 | 1.02 d1 | 0.079 | *** |
| Frozen stored # | 1.19 a2 | 0.51 c2 | 1.31 a2 | 1.18 a2 | 0.82 b2 | 0.067 | *** |
| SEM | 0.075 | 0.037 | 0.054 | 0.054 | 0.076 | ||
| p-value | *** | *** | *** | ** | ** |
| LRI | CON | ASC | LBPS | MBPS | HBPS | SEM | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Aldehydes and ketones | 369.0 c | 215.0 d | 643.5 a | 542.2 b | 543.1 b | 35.30 | *** | |
| Hexanal | 807 | 237.7 c | 134.1 d | 424.9 a | 360.1 b | 342.3 b | 20.76 | *** |
| 2-Heptanona | 901 | 2.7 c | 1.0 d | 7.0 a | 4.7 b | 3.8 bc | 0.41 | ** |
| Heptanal | 907 | 32.7 c | 20.9 d | 53.7 a | 46.1 b | 46.55 ab | 3.83 | * |
| Octanal | 1007 | 23.8 c | 12.5 d | 47.1 a | 35.5 b | 39.4 ab | 4.19 | * |
| 2-Octenal | 1078 | 1.2 | 1.4 | 2.7 | 2.1 | 2.6 | 0.32 | NS |
| Nonanal | 1108 | 64.5 b | 41.8 c | 98.7 a | 84.8 a | 96.2 a | 6.05 | ** |
| Methyl-2-nonenal | 1226 | 2.7 b | 1.9 c | 3.4 ab | 3.1 ab | 3.8 a | 0.23 | * |
| 2-Decenal | 1287 | 0.9 | 0.4 | 2.3 | 2.6 | 4.2 | 0.46 | NS |
| Alcohols | 23.3 | 8.5 | 33.1 | 28.7 | 33.1 | 3.09 | NS | |
| Pentanol | 770 | 13.5 | 4.8 | 19.4 | 15.9 | 19.0 | 1.72 | NS |
| Octanol | 1087 | 9.3 | 3.7 | 12.5 | 11.0 | 11.9 | 1.26 | NS |
| Furans | 161.7 c | 95.1 d | 250.7 a | 202.3 b | 204.0 b | 13.52 | ** | |
| 2-Butylfuran | 893 | 3.2 a | 1.7 b | 4.0 a | 3.8 a | 3.0 a | 0.28 | * |
| 2-Pentyl | 989 | 151.6 c | 89.3 d | 235.5 a | 188.2 bc | 190.6 b | 12.35 | ** |
| 2-Hexylfuran | 1091 | 2.6 | 1.5 | 3.9 | 3.8 | 3.8 | 0.39 | NS |
| 2-Heptylfuran | 1203 | 1.9 b | 0.8 c | 2.7 a | 2.3 ab | 2.9 a | 0.25 | * |
| Aliphatic hydrocarbons | 18.6 b | 11.0 c | 24.2 a | 20.8 ab | 23.2 a | 1.73 | * | |
| Octane | 800 | 11.4 b | 6.0 c | 15.1 a | 13.5 ab | 14.8 ab | 1.18 | * |
| Terpene compounds | 300.1 | 280.5 | 269.0 | 239.5 | 255.1 | 8.47 | NS | |
| α-Pinene | 932 | 30.5 | 32.9 | 28.7 | 23.7 | 26.8 | 1.58 | NS |
| Camphene | 953 | 4.6 a | 3.5 b | 3.6 b | 3.2 b | 3.4 b | 0.16 | * |
| β-Pinene | 986 | 27.1 | 24.4 | 23.7 | 20.6 | 21.8 | 0.77 | NS |
| Myrcene | 991 | 7.0 | 7.7 | 6.3 | 5.6 | 6.3 | 0.26 | NS |
| α-Phellandrene | 993 | 3.7 | 7.1 | 2.9 | 3.7 | 3.3 | 1.06 | NS |
| δ-Carene | 1008 | 119.4 | 108.5 | 103.2 | 97.7 | 100.0 | 3.50 | NS |
| p-Cymene | 1026 | 12.7 | 8.2 | 10.6 | 10.1 | 11.1 | 0.52 | NS |
| Limonene | 1028 | 85.5 | 78.3 | 78.6 | 66.6 | 74.1 | 2.36 | NS |
| β-Caryophyllene | 1447 | 4.5 b | 6.0 a | 3.9 b | 3.5 b | 3.6 b | 0.22 | ** |
| Sulphur compounds | 7.7 ab | 5.6 b | 10.3 a | 8.9 a | 8.7 a | 0.48 | * | |
| Dimethyl disulphide | 755 | 7.5 ab | 5.6 b | 9.8 a | 8.4 a | 8.4 a | 0.45 | * |
| Total sum of volatiles | 883.9 c | 618.2 d | 1234.9 a | 1045.9 b | 1071.0 b | 54.14 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carballo, D.E.; Caro, I.; Gallego, C.; González, A.R.; Giráldez, F.J.; Andrés, S.; Mateo, J. Banana Pseudo-Stem Increases the Water-Holding Capacity of Minced Pork Batter and the Oxidative Stability of Pork Patties. Foods 2021, 10, 2173. https://doi.org/10.3390/foods10092173
Carballo DE, Caro I, Gallego C, González AR, Giráldez FJ, Andrés S, Mateo J. Banana Pseudo-Stem Increases the Water-Holding Capacity of Minced Pork Batter and the Oxidative Stability of Pork Patties. Foods. 2021; 10(9):2173. https://doi.org/10.3390/foods10092173
Chicago/Turabian StyleCarballo, Diego E., Irma Caro, Cristina Gallego, Ana Rebeca González, Francisco Javier Giráldez, Sonia Andrés, and Javier Mateo. 2021. "Banana Pseudo-Stem Increases the Water-Holding Capacity of Minced Pork Batter and the Oxidative Stability of Pork Patties" Foods 10, no. 9: 2173. https://doi.org/10.3390/foods10092173
APA StyleCarballo, D. E., Caro, I., Gallego, C., González, A. R., Giráldez, F. J., Andrés, S., & Mateo, J. (2021). Banana Pseudo-Stem Increases the Water-Holding Capacity of Minced Pork Batter and the Oxidative Stability of Pork Patties. Foods, 10(9), 2173. https://doi.org/10.3390/foods10092173