Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotics Bacterial Cell
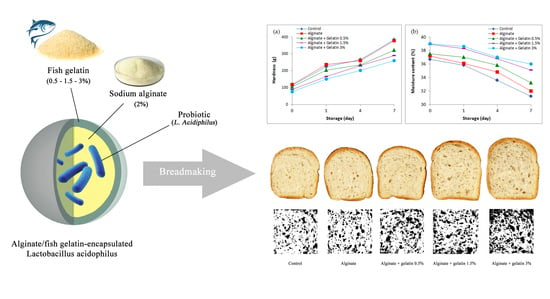
2.2. Encapsulation in Alginate/Fish Gelatin Matrix
2.3. Breadmaking
2.4. Viable Cell Counts of L. acidophilus
2.5. Encapsulation Efficiency, Particle Size, and Zeta Potential of Capsules
2.6. Determination of Hardness and Staling Rate
2.7. Determination of Moisture Content
2.8. Oven Spring and Specific Volume Index
2.9. Internal Texture Structure Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency, Particle Size, and Zeta Potential of Capsules
3.2. Viability of Encapsulated L. acidophilus during Baking and Storage
3.3. Hardness and Staling Rate
3.4. Moisture Content
3.5. Oven Spring and Specific Volume
3.6. Internal Texture Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef]
- Vallejo-Castillo, V.; Rodríguez-Stouvenel, A.; Martínez, R.; Bernal, C. Development of alginate-pectin microcapsules by the extrusion for encapsulation and controlled release of polyphenols from papaya (Carica papaya L.). J. Food Biochem. 2020, 44, e13331. [Google Scholar] [CrossRef]
- De Souza, E.L.; De Albuquerque, T.M.R.; Santos, A.; Massa, N.M.L.; Alves, J.L.D.B. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1645–1659. [Google Scholar] [CrossRef]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.D.; Boom, R.M.; Schutyser, M.A. Survival of encapsulated Lactobacillus plantarum during isothermal heating and bread baking. LWT 2018, 93, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yogurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.; Singh, K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Fiori, F.; Rovera, C.; Farris, S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocoll. 2020, 109, 106111. [Google Scholar] [CrossRef]
- Goudoulas, T.; Germann, N. Phase transition kinetics and rheology of gelatin-alginate mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Li, D.; Guo, L.; Su, X.; Zhang, Y.; Wu, F.; Xu, X. Effect of pigskin-originated gelatin on properties of wheat flour dough and bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Tebben, L.; Li, Y. Effect of xanthan gum on dough properties and bread qualities made from whole wheat flour. Cereal Chem. J. 2018, 96, 263–272. [Google Scholar] [CrossRef]
- Moghanjougi, Z.M.; Bari, M.R.; Khaledabad, M.A.; Amiri, S.; Almasi, H. Microencapsulation of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 in pectin and sodium alginate: A comparative study on viability, stability, and structure. Food Sci. Nutr. 2021, 9, 5103–5111. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Ananingsih, V.K.; Zhou, W.; Chen, X.D. A study on Bifidobacterium lactis Bb12 viability in bread during baking. J. Food Eng. 2013, 122, 33–37. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of hyssop essential oil in chitosan-pea protein isolate nano-complex. LWT 2021, 144, 111254. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Rashidi-Alavijeh, S.; Ahmadi, S. Encapsulation of Lactobacillus casei in quince seed gum-alginate beads to produce a functional synbiotic drink powder by agro-industrial by-products and freeze-drying. Food Hydrocoll. 2021, 120, 106895. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; AACCI: St. Paul, MN, USA, 2010. [Google Scholar]
- Giri, N.A.; Sakhale, B.K. Optimization of whey protein concentrate and psyllium husk for the development of protein-fiber rich orange fleshed sweet potato (Ipomoea batatas L.) bread by using response surface methodology. J. Food Meas. Charact. 2019, 14, 425–437. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B. Optimization of bread firmness, specific loaf volume and sensory acceptability of bread with soluble fiber and different water levels. J. Cereal Sci. 2016, 70, 186–191. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ayadi, D.; Bejar, W.; Bejar, S.; Chouayekh, H.; Ben Salah, R. Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int. J. Biol. Macromol. 2014, 64, 84–89. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, T.N.; El-Sayed, H. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Wang, W.; Liu, G.; Ge, W.; Zhang, M.; Yue, B.; Kong, M. Microencapsulation of Lactobacillus casei BNCC 134415 under lyophilization enhances cell viability during cold storage and pasteurization, and in simulated gastrointestinal fluids. LWT 2019, 116, 108521. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Ibarz, A. Modified mung bean protein: Optimization of microwave-assisted phosphorylation and its functional and structural characterizations. LWT 2021, 151, 112119. [Google Scholar] [CrossRef]
- Perdana, J.; Bereschenko, L.; Fox, M.B.; Kuperus, J.H.; Kleerebezem, M.; Boom, R.M.; Schutyser, M.A. Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: Comparing single droplet drying to spray and freeze drying. Food Res. Int. 2013, 54, 1351–1359. [Google Scholar] [CrossRef]
- Thang, T.D.; Quyen, L.T.H.; Hang, H.T.T.; Luan, N.T.; KimThuy, D.T.; Lieu, D.M. Survival Survey of Lactobacillus acidophilus in additional probiotic bread. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Yang, Y.; Fu, N.; Qin, Q.; Zhang, L.; Chen, X.D. Calcium-aggregated milk: A potential new option for improving the viability of lactic acid bacteria under heat stress. Food Bioprocess Technol. 2014, 7, 3147–3155. [Google Scholar] [CrossRef]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M. Different hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- Collar, C.; Martínez, J.C.; Rosell, C.M. Lipid binding of fresh and stored formulated wheat breads. Relationships with dough and bread technological performance. Food Sci. Technol. Int. 2001, 7, 501–510. [Google Scholar] [CrossRef]
- Rosell, C.; Rojas, J.; De Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Walsh, D.; Valverde, J.; Hayes, M. Chitosan-containing bread made using marine shellfishery byproducts: Functional, bioactive, and quality assessment of the end product. J. Agric. Food Chem. 2013, 61, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Shittu, T.A.; Aminu, R.A.; Abulude, E.O. Functional effects of xanthan gum on composite cassava-wheat dough and bread. Food Hydrocoll. 2009, 23, 2254–2260. [Google Scholar] [CrossRef]
- Lamacchia, C.; Landriscina, L.; Severini, C.; Caporizzi, R.; Derossi, A. Characterizing the rheological and bread-making properties of wheat flour treated by “Gluten FriendlyTM” technology. Foods 2021, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Turbin-Orger, A.; Boller, E.; Chaunier, L.; Chiron, H.; Della Valle, G.; Réguerre, A.-L. Kinetics of bubble growth in wheat flour dough during proofing studied by computed X-ray micro-tomography. J. Cereal Sci. 2012, 56, 676–683. [Google Scholar] [CrossRef]



| Sample | Encapsulation Efficiency (%) | Particle Size (µm) | Zeta Potential (mV) |
|---|---|---|---|
| Alginate | 92.30 ± 3.18 d | 389.8 ± 20.9 d | −13.5 ± 2.2 c |
| Alginate + Gelatin 0.5% | 95.42 ± 1.36 c | 569.3 ± 36.9 c | −17.3 ± 1.4 b |
| Alginate + Gelatin 1.5% | 96.33 ± 2.45 b | 682.1 ± 0.05 b | −21.0 ± 2.5 a |
| Alginate + Gelatin 3% | 98.68 ± 1.97 a | 736.5 ± 0.04 a | −21.6 ± 3.1 a |
| Sample | Storage (Day) | ||||
|---|---|---|---|---|---|
| Dough | After Baking | 1 | 4 | 7 | |
| Control | 9.40 ± 0.29 a,A | 2.89 ± 0.13 c,D | 2.74 ± 0.14 c,D | 3.45 ± 0.16 c,C | 4.59 ± 0.11 c,B |
| Alginate | 9.22 ± 0.24 b,A | 4.43 ± 0.14 b,C | 4.36 ± 0.26 b,C | 5.28 ± 0.25 b,B | 5.44 ± 0.22 b,B |
| Alginate + Gelatin 0.5% | 9.35 ± 0.31 a,A | 4.37 ± 0.19 b,D | 4.20 ± 0.28 b,D | 5.15 ± 0.26 b,C | 5.57 ± 0.15 b,B |
| Alginate + Gelatin 1.5% | 9.19 ± 0.12 b,A | 5.21 ± 0.25 a,D | 5.19 ± 0.15 a,D | 6.25 ± 0.12 a,C | 7.82 ± 0.31 a,B |
| Alginate + Gelatin 3% | 9.33 ± 0.14 a,A | 5.38 ± 0.34 a,D | 5.25 ± 0.24 a,D | 6.42 ± 0.16 a,C | 7.66 ± 0.24 a,B |
| Sample | Porosity (%) | Cell Average Area (mm2) | Cell Density (Cells/cm2) |
|---|---|---|---|
| Control | 30.49 ± 3.11 d | 0.69 ± 0.06 d | 37.36± 4.24 c |
| Alginate | 31.25 ± 1.89 d | 0.60 ± 0.03 c | 41.96 ± 2.16 b |
| Alginate + Gelatin 0.5% | 34.73 ± 2.05 c | 0.55 ± 0.07 b,c | 43.01 ± 5.50 b |
| Alginate + Gelatin 1.5% | 38.19 ± 3.64 b | 0.51 ± 0.05 b | 47.83 ± 2.95 a |
| Alginate + Gelatin 3% | 41.25 ± 3.37 a | 0.43 ± 0.04 a | 48.20 ± 3.46 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Mousavi Khanegah, A. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods 2021, 10, 2215. https://doi.org/10.3390/foods10092215
Hadidi M, Majidiyan N, Jelyani AZ, Moreno A, Hadian Z, Mousavi Khanegah A. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods. 2021; 10(9):2215. https://doi.org/10.3390/foods10092215
Chicago/Turabian StyleHadidi, Milad, Nava Majidiyan, Aniseh Zarei Jelyani, Andrés Moreno, Zahra Hadian, and Amin Mousavi Khanegah. 2021. "Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage" Foods 10, no. 9: 2215. https://doi.org/10.3390/foods10092215
APA StyleHadidi, M., Majidiyan, N., Jelyani, A. Z., Moreno, A., Hadian, Z., & Mousavi Khanegah, A. (2021). Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods, 10(9), 2215. https://doi.org/10.3390/foods10092215