Development and Validation of a Simple Method to Quantify Contents of Phospholipids in Krill Oil by Fourier-Transform Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. FT-IR Spectroscopy
2.4. Validation of Analytical Method
2.5. 31P NMR Spectroscopy
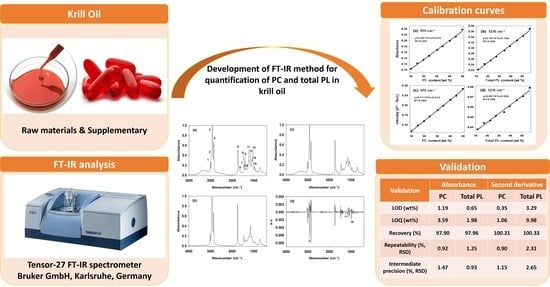
3. Results and Discussion
3.1. FT-IR Spectrum of Krill Oil
3.2. Development of FT-IR Quantification Method
3.2.1. Calibration Curves
3.2.2. Method Validation
3.3. Application of Proposed FT-IR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmmed, F.; Fuller, I.D.; Killeen, D.P.; Fraser-Miller, S.J.; Gordon, K.C. Raman and Infrared Spectroscopic Data Fusion Strategies for Rapid, Multicomponent Quantitation of Krill Oil Compositions. J. Food Sci. Technol. 2021, 1, 570–578. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.; Jinap, S.; Saari, N.; Jahurul, H.; Abbas, K.; Norulaini, N. PUFAs in fish: Extraction, fractionation, importance in health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; He, F.; Wang, X.; Wang, X.; Jin, Q.; Jin, J. Diverse Krill Lipid Fractions Differentially Reduce LPS-Induced Inflammatory Markers in RAW264.7 Macrophages In Vitro. Foods 2021, 10, 2887. [Google Scholar] [CrossRef]
- Araujo, P.; Zhu, H.; Breivik, J.F.; Hjelle, J.I.; Zeng, Y. Determination and structural elucidation of triacylglycerols in krill oil by chromatographic techniques. Lipids 2014, 49, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects--a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Kwantes, J.M.; Grundmann, O. A brief review of krill oil history, research, and the commercial market. J. Diet. Suppl. 2015, 12, 23–35. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Commission, C.A. Codex329-2017; Standard for Fish Oils., Codex Alimentarius Commission: Rome, Italy, 2017. [Google Scholar]
- Commission, C.A. Codex234-1999; Recommended Methods of Analysis and Sampling., Codex Alimentarius Commission: Rome, Italy, 1999. [Google Scholar]
- Schiller, J.; Muller, M.; Fuchs, B.; Arnold, K.; Huster, D. 31P NMR spectroscopy of phospholipids: From micelles to membranes. Curr. Anal. Chem. 2007, 3, 283–301. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Shen, J.; Kim, J.H.; Cho, J.Y.; Hong, W.S.; Meda, V.; Reaney, M.J.T. Aquafaba from Korean Soybean II: Physicochemical Properties and Composition Characterized by NMR Analysis. Foods 2021, 10, 2589. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Camin, F.; Bontempo, L.; Proto, A. Application of 13C Quantitative NMR Spectroscopy to Isotopic Analyses for Vanillin Authentication Source. Foods 2021, 10, 2635. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Myoung, S.; Seo, Y.; Ahn, S. Quantitative NMR as a Versatile Tool for the Reference Material Preparation. Magnetochemistry 2021, 7, 15. [Google Scholar] [CrossRef]
- Meng, X.; Sedman, J.; van de Voort, F.R. Improving the determination of moisture in edible oils by FTIR spectroscopy using acetonitrile extraction. Food Chem. 2012, 135, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.L.; Cummings, B.S. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed. Chromatogr. 2006, 20, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Cirillo, G.; Vinci, G.; Picci, N. Determination of Phospholipids in Food Samples. Food Rev. Int. 2012, 28, 1–46. [Google Scholar] [CrossRef]
- Van de Voort, F. Fourier transform infrared spectroscopy applied to food analysis. Food Res. Int. 1992, 25, 397–403. [Google Scholar] [CrossRef]
- Al-Alawi, A.; van de Voort, F.R.; Sedman, J.; Ghetler, A. Automated FTIR Analysis of Free Fatty Acids or Moisture in Edible Oils. J. Lab. Autom. 2016, 11, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Influence of fat extraction methods on the peroxide value in infant formulas. Food Res. Int. 2012, 48, 584–591. [Google Scholar] [CrossRef]
- Oussama, A.; Elabadi, F.; Devos, O. Analysis of Argan Oil Adulteration Using Infrared Spectroscopy. Spectrosc. Lett 2012, 45, 458–463. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Sherazi, S.T.H.; Kandhro, A.; Mahesar, S.A.; Bhanger, M.I.; Talpur, M.Y.; Arain, S. Application of transmission FT-IR spectroscopy for the trans fat determination in the industrially processed edible oils. Food Chem. 2009, 114, 323–327. [Google Scholar] [CrossRef]
- Yu, X.; Du, S.; van de VOORT, F.R.; Yue, T.; Li, Z. Automated and simultaneous determination of free fatty acids and peroxide values in edible oils by FTIR spectroscopy using spectral reconstitution. Anal. Sci. 2009, 25, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Spyrelli, E.D.; Papachristou, C.K.; Nychas, G.-J.E.; Panagou, E.Z. Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis. Foods 2021, 10, 2723. [Google Scholar] [CrossRef]
- AOCS, F.D. Official methods and recommended practices of the American Oil Chemists’ Society. J. Am. Oil. Chem. Soc. 1998, 5, 2–93. [Google Scholar]
- Park, S.M.; Yu, H.Y.; Chun, H.S.; Kim, B.H.; Ahn, S. A Second Derivative Fourier-Transform Infrared Spectroscopy Method to Discriminate Perilla Oil Authenticity. J. Oleo Sci. 2019, 68, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Nzai, J.; Proctor, A. Soy lecithin phospholipid determination by fourier transform infrared spectroscopy and the acid digest/arseno-molybdate method: A comparative study. J. Am. Oil Chem. Soc. 1999, 76, 61–66. [Google Scholar] [CrossRef]
- Szłyk, E.; Szydłowska-Czerniak, A.; Kowalczyk-Marzec, A. NIR spectroscopy and partial least-squares regression for determination of natural α-tocopherol in vegetable oils. J. Agric. Food Chem. 2005, 53, 6980–6987. [Google Scholar] [CrossRef] [PubMed]
- Whittinghill, J.; Norton, J.; Proctor, A. Stability determination of soy lecithin-based emulsions by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 37–42. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Narhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of Fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, B.; Kohler, A. Optimizing Savitzky-Golay parameters for improving spectral resolution and quantification in infrared spectroscopy. Appl. Spectrosc. 2013, 67, 892–902. [Google Scholar] [CrossRef] [Green Version]
- USP-NF(43) Krill Oil Monograph, 38th ed.; The United States Pharmacopeial Convention, USP 43-NF38; The United States Parmacopeial Convention, Inc.: North Bethesda, MD, USA, 2020; Volume 3, pp. 5100–5111.
- Burri, L.; Hoem, N.; Monakhova, Y.B.; Diehl, B.W.K. Fingerprinting Krill Oil by 31P, 1H and 13C NMR Spectroscopies. J. Am. Oil. Chem. Soc. 2016, 93, 1037–1049. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Diehl, B.W.K. Automated multicomponent phospholipid analysis using 31P NMR spectroscopy: Example of vegetable lecithin and krill oil. Anal. Biochem. 2018, 410, 7891–7900. [Google Scholar] [CrossRef] [PubMed]
- Szydlowskaczerniak, A. MIR spectroscopy and partial least-squares regression for determination of phospholipids in rapeseed oils at various stages of technological process. Food Chem. 2007, 105, 1179–1187. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Haider, J.; Majeed, H.; Williams, P.A.; Safdar, W.; Zhong, F. Formation of chitosan nanoparticles to encapsulate krill oil (Euphausia superba) for application as a dietary supplement. Food Hydrocoll. 2017, 63, 27–34. [Google Scholar] [CrossRef]
- Yan, C.; Huang, W.; Xu, J.; Yang, H.; Zhang, Y.; Bahia, H.U. Quantification of re-refined engine oil bottoms (REOB) in asphalt binder using ATR-FTIR spectroscopy associated with partial least squares (PLS) regression. Road Mater. Pavement Des. 2020, 1–15. [Google Scholar] [CrossRef]
- Kuligowski, J.; Quintas, G.; Esteve-Turrillas, F.A.; Garrigues, S.; de la Guardia, M. On-line gel permeation chromatography-attenuated total reflectance-Fourier transform infrared determination of lecithin and soybean oil in dietary supplements. J. Chromatogr. A 2008, 1185, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Pan, Q.; Ding, Y.; Jiang, L. Rapid determination of phospholipid content of vegetable oils by FTIR spectroscopy combined with partial least-square regression. Food Chem. 2014, 147, 272–278. [Google Scholar] [CrossRef]
- Schulz, H.; Schrader, B.; Quilitzsch, R.; Steuer, B. Quantitative analysis of various citrus oils by ATR/FT-IR and NIR-FT Raman spectroscopy. Appl. Spectrosc. 2002, 56, 117–124. [Google Scholar] [CrossRef]



| Peak Variable | Wavenumber (cm−1) | Functional Group | Mode of Vibration |
|---|---|---|---|
| I1 | 3010 | =C–H (cis-) | Stretching |
| I2 | 2958 | –C–H (CH3) | Stretching (asym) |
| I3 | 2924 | –C–H (CH2) | Stretching (asym) |
| I4 | 2853 | –C–H (CH2) | Stretching (sym) |
| I5 | 1745 | –C=O (ester) | Stretching |
| I6 | 1654 | –C=C– (cis-) | Stretching |
| I7 | 1466 | –C–H (CH2, CH3) | Bending (scissoring) |
| I8 | 1419 | =C–H (cis-) | Bending (rocking) |
| I9 | 1395 | =C–H (cis-) | Bending |
| I10 | 1377 | –C–H (CH3) | Bending (sym) |
| I11 | 1236 | PO2− (diester) | Stretching (asym) |
| I12 | 1167 | –C–O–P (ester) | Stretching (sym) |
| I13 | 1090 | PO2− (diester) | Stretching (sym) |
| I14 | 1060 | –C–O–P (ester) | Stretching (asym) |
| I15 | 970 | –N–(CH3)3 | Stretching (asym) |
| KERRYPNX | Absorbance | Second-Derivative | ||
|---|---|---|---|---|
| PC | Total PL | PC | Total PL | |
| LOD (wt%) | 1.19 | 0.65 | 0.35 | 3.29 |
| LOQ (wt%) | 3.59 | 1.98 | 1.06 | 9.98 |
| Recovery (%) | 97.90 | 97.96 | 100.21 | 100.33 |
| Repeatability (%, RSD) | 0.92 | 1.25 | 0.90 | 2.31 |
| Intermediate precision (%, RSD) | 1.47 | 0.93 | 1.15 | 2.65 |
| Test Sample | PL Contents (wt%) ± Standard Deviation | |||||
|---|---|---|---|---|---|---|
| FT-IR Method | 31P NMR Method | |||||
| Absorbance | Second-Derivative | |||||
| PC | Total PL | PC | Total PL | PC | Total PL | |
| Mixture 1 | 19.25 ± 0.31 | 22.00 ± 0.42 | 19.66 ± 0.06 | 22.89 ± 0.65 | 20.02 ± 0.02 | 22.68 ± 0.01 |
| Mixture 2 | 22.44 ± 0.19 | 25.76 ± 0.10 | 23.14 ± 0.06 | 25.39 ± 0.81 | 22.04 ± 0.31 | 25.10 ± 0.24 |
| Mixture 3 | 33.03 ± 0.56 | 37.92 ± 0.63 | 33.56 ± 0.28 | 37.89 ± 0.19 | 32.18 ± 1.08 | 36.56 ± 1.22 |
| Mixture 4 | 36.10 ± 0.65 | 41.11 ± 0.72 | 36.83 ± 0.61 | 41.90 ± 0.41 | 36.71 ± 0.33 | 41.84 ± 0.33 |
| Mixture 5 | 43.78 ± 0.44 | 49.99 ± 0.43 | 43.96 ± 0.03 | 50.54 ± 1.20 | 44.46 ± 0.11 | 50.74 ± 0.07 |
| Supplement 1 | 50.95 ± 0.64 | 55.29 ± 0.69 | 50.01 ± 0.19 | 56.41 ± 1.29 | 49.39 ± 0.69 | 56.12 ± 0.78 |
| Supplement 2 | 53.38 ± 0.37 | 54.30 ± 0.54 | 47.10 ± 0.69 | 52.58 ± 0.96 | 48.05 ± 1.53 | 52.14 ± 1.66 |
| Supplement 3 | 35.89 ± 0.26 | 38.82 ± 1.03 | 34.95 ± 0.77 | 35.89 ± 1.17 | 35.21 ± 1.55 | 39.59 ± 1.74 |
| Supplement 4 | 33.82 ± 1.35 | 38.39 ± 1.50 | 32.25 ± 0.77 | 34.12 ± 0.67 | 31.13 ± 1.85 | 35.50 ± 2.11 |
| Supplement 5 | 37.94 ± 2.24 | 41.43 ± 2.12 | 34.94 ± 0.52 | 37.70 ± 0.37 | 34.06 ± 0.96 | 38.71 ± 1.09 |
| Supplement 6 | 41.75 ± 0.58 | 42.64 ± 0.58 | 40.28 ± 0.77 | 44.33 ± 0.85 | 40.49 ± 0.10 | 45.23 ± 0.11 |
| Supplement 7 | 44.94 ± 1.32 | 48.05 ± 1.27 | 37.13 ± 1.13 | 39.17 ± 0.79 | 38.73 ± 1.10 | 44.31 ± 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-E.; Yu, H.-Y.; Ahn, S. Development and Validation of a Simple Method to Quantify Contents of Phospholipids in Krill Oil by Fourier-Transform Infrared Spectroscopy. Foods 2022, 11, 41. https://doi.org/10.3390/foods11010041
Park S-E, Yu H-Y, Ahn S. Development and Validation of a Simple Method to Quantify Contents of Phospholipids in Krill Oil by Fourier-Transform Infrared Spectroscopy. Foods. 2022; 11(1):41. https://doi.org/10.3390/foods11010041
Chicago/Turabian StylePark, Se-Eun, Hyo-Yeon Yu, and Sangdoo Ahn. 2022. "Development and Validation of a Simple Method to Quantify Contents of Phospholipids in Krill Oil by Fourier-Transform Infrared Spectroscopy" Foods 11, no. 1: 41. https://doi.org/10.3390/foods11010041
APA StylePark, S. -E., Yu, H. -Y., & Ahn, S. (2022). Development and Validation of a Simple Method to Quantify Contents of Phospholipids in Krill Oil by Fourier-Transform Infrared Spectroscopy. Foods, 11(1), 41. https://doi.org/10.3390/foods11010041