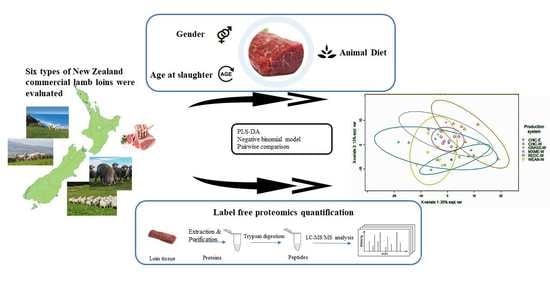
Proteomic Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Meat Sampling and Storage
2.3. Protein Extraction
2.4. Protein Digestion
2.5. Mass Spectrometric Analysis
2.6. Protein Identification
2.7. Label Free Quantification
2.8. Statistical Analysis
3. Results
3.1. Differences in Protein Profiles in Meat from Lambs Reared in Six Forage Production Systems
3.2. Protein Comparison between the Meat Proteome from Ewe and Wether Lambs Grazed on Chicory (Chic-E vs. Chic-W)
3.3. Protein Comparison between the Meat Proteome from Wether Lambs Grazed on Perennial Ryegrass and Chicory (Grass-W vs. Chic-W)
3.4. Protein Comparison between Meat from Wether Lambs Slaughtered at 4-Months of Age and 6- to 8-Months of Age (Wean-W vs. Chic-W)
4. Discussion
4.1. Proteome Abundance Differences Due to Gender (Chic-E vs. Chic-W)
4.2. Proteome Abundance Differences Due to Diet (Grass-W vs. Chic-W)
4.3. Proteome Abundance Differences Due to Age (Wean-W vs. Chic-W)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Beef + Lamb New Zealand Ltd. Beef+lamb New Zealand New Season Outlook 2020–2021. 2020. Available online: https://beeflambnz.com/sites/default/files/news-docs/New-Season-Outlook-21-22-summary.pdf (accessed on 6 March 2022).
- Jiang, H.; Wang, Z.; Ma, Y.; Qu, Y.; Lu, X.; Guo, H.; Luo, H. Effect of Dietary Lycopene Supplementation on Growth Performance, Meat Quality, Fatty Acid Profile and Meat Lipid Oxidation in Lambs in Summer Conditions. Small Rumin. Res. 2014, 131, 99–106. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Mortimer, S.I. Effect of Genotype, Gender and Age on Sheep Meat Quality and a Case Study Illustrating Integration of Knowledge. Meat Sci. 2014, 98, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Schreurs, N.M.; Johnson, P.L.; Corner-Thomas, R.A.; Agnew, M.P.; Silcock, P.; Eyres, G.T.; Maclennan, G.; Realini, C.E. Carcass Characteristics and Meat Quality of Commercial Lambs Reared in Different Forage Systems. Livest. Sci. 2020, 232, 103908. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Soulat, J.; Monteils, V. Clustering of Sensory Eating Qualities of Beef: Consistencies and Differences within Carcass, Muscle, Animal Characteristics and Rearing Factors. Livest. Sci. 2018, 214, 245–258. [Google Scholar] [CrossRef]
- Mashele, G.A.; Parker, M.E.; Schreurs, N.M. Effect of Slaughter Age between 5 to 14 Months of Age on the Quality of Sheep Meat. Proc. N. Z. Soc. Anim. Prod. 2017, 77, 177–180. [Google Scholar]
- Picard, B.; Gagaoua, M.; Al Jammas, M.; Bonnet, M. Beef Tenderness and Intramuscular Fat Proteomic Biomarkers: Effect of Gender and Rearing Practices. J. Proteom. 2019, 200, 1–10. [Google Scholar] [CrossRef]
- Clerens, S.; Thomas, A.; Gathercole, J.; Plowman, J.E.; Yu, T.Y.; Grosvenor, A.J.; Haines, S.R.; Dobbie, P.; Taukiri, K.; Rosenvold, K.; et al. Proteomic and Peptidomic Differences and Similarities between Four Muscle Types from New Zealand Raised Angus Steers. Meat Sci. 2016, 121, 53–63. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Geesink, G.H. Contribution of Postmortem Muscle Biochemistry to the Delivery of Consistent Meat Quality with Particular Focus on the Calpain System. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T. The Role of Intramuscular Connective Tissue in Meat Texture. Anim. Sci. J. 2010, 81, 21–27. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Meta-Proteomics for the Discovery of Protein Biomarkers of Beef Tenderness: An Overview of Integrated Studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current Research in Meat Color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Paredi, G.; Raboni, S.; Bendixen, E.; Almeida, A.M.; Mozzarelli, A. “Muscle to Meat” Molecular Events and Technological Transformations: The Proteomics Insight. J. Proteom. 2012, 75, 4275–4289. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, X.; Zhang, D.; Liu, Y. Comparison of Protein Differences between High- and Low-Quality Goat and Bovine Parts Based on ITRAQ Technology. Food Chem. 2019, 289, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Daroit, D.J.; Brandelli, A. Implications of Skeletal Muscle Creatine Kinase to Meat Quality. J. Anim. Feed Sci. 2008, 17, 285–294. [Google Scholar] [CrossRef]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Proteomic Investigation of Protein Profile Changes and Amino Acid Residue-Level Modification in Cooked Lamb Longissimus Thoracis et Lumborum: The Effect of Roasting. Meat Sci. 2016, 119, 80–88. [Google Scholar] [CrossRef]
- Wessel, D.; Flügge, U.I. A Method for the Quantitative Recovery of Protein in Dilute Solution in the Presence of Detergents and Lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Lametsch, R.; Karlsson, A.; Rosenvold, K.; Andersen, H.J.; Roepstorff, P.; Bendixen, E. Postmortem Proteome Changes of Porcine Muscle Related to Tenderness. J. Agric. Food Chem. 2003, 51, 6992–6997. [Google Scholar] [CrossRef]
- Di Luca, A.; Elia, G.; Hamill, R.; Mullen, A.M. 2D DIGE Proteomic Analysis of Early Post Mortem Muscle Exudate Highlights the Importance of the Stress Response for Improved Water-Holding Capacity of Fresh Pork Meat. Proteomics 2013, 13, 1528–1544. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Wiklund, E.; Young, O.A. Small Heat Shock Proteins and Their Role in Meat Tenderness: A Review. Meat Sci. 2014, 96, 26–40. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Xu, X.; Xu, W. Comparative Proteomic Analysis of Proteins Associated with Water Holding Capacity in Goose Muscles. Food Res. Int. 2019, 116, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Beldarrain, L.R.; Aldai, N.; Picard, B.; Sentandreu, E.; Navarro, J.L.; Sentandreu, M.A. Use of Liquid Isoelectric Focusing (OFFGEL) on the Discovery of Meat Tenderness Biomarkers. J. Proteom. 2018, 183, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Geesink, G.H. Protein Degradation Post Mortem and Tenderisation. Appl. Muscle Biol. Meat Sci. 2009, 149–173. [Google Scholar]
- Kim, G.D.; Yang, H.S.; Jeong, J.Y. Intramuscular Variations of Proteome and Muscle Fiber Type Distribution in Semimembranosus and Semitendinosus Muscles Associated with Pork Quality. Food Chem. 2018, 244, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagaoua, M.; Terlouw, E.M.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular Signatures of Beef Tenderness: Underlying Mechanisms Based on Integromics of Protein Biomarkers from Multi-Platform Proteomics Studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef]
- Kim, N.K.; Cho, S.; Lee, S.H.; Park, H.R.; Lee, C.S.; Cho, Y.M.; Choy, Y.H.; Yoon, D.; Im, S.K.; Park, E.W. Proteins in Longissimus Muscle of Korean Native Cattle and Their Relationship to Meat Quality. Meat Sci. 2008, 80, 1068–1073. [Google Scholar] [CrossRef]
- Hamelin, M.; Sayd, T.; Chambon, C.; Bouix, J.; Bibé, B.; Milenkovic, D.; Leveziel, H.; Georges, M.; Clop, A.; Marinova, P.; et al. Proteomic Analysis of Ovine Muscle Hypertrophy. J. Anim. Sci. 2006, 84, 3266–3276. [Google Scholar] [CrossRef] [Green Version]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and Lipid Oxidation Interactions: Mechanistic Bases and Control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Grossmann, J.; Fortes, C.; Kilminster, T.; Scanlon, T.; Milton, J.; Greeff, J.; Oldham, C.; Nanni, P.; Almeida, A.M. The Sheep (Ovis Aries) Muscle Proteome: Decoding the Mechanisms of Tolerance to Seasonal Weight Loss Using Label-Free Proteomics. J. Proteom. 2017, 161, 57–67. [Google Scholar] [CrossRef]
- De Oliveira Monteschio, J.; Burin, P.C.; Leonardo, A.P.; Fausto, D.A.; Da Silva, A.L.A.; De Almeida Ricardo, H.; Da Silva, M.C.; De Souza, M.R.; De Vargas, F.M. Different Physiological Stages and Breeding Systems Related to the Variability of Meat Quality of Indigenous Pantaneiro Sheep. PLoS ONE 2018, 13, e0191668. [Google Scholar] [CrossRef] [Green Version]
- Young, O.A.; Hogg, B.W.; Mortimer, B.J.; Waller, J.E. Collagen in Two Muscles of Sheep Selected for Weight as Yearlings. N. Z. J. Agric. Res. 1993, 36, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, D.L.; Allingham, P.G.; Colgrave, M.; Van De Ven, R.J. Interrelationship between Measures of Collagen, Compression, Shear Force and Tenderness. Meat Sci. 2013, 95, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.R.; Rogers, R.W.; Althen, T.G. The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002, 18, 107–111. [Google Scholar] [CrossRef]
- Somasiri, S.C.; Kenyon, P.R.; Kemp, P.D.; Morel, P.C.H.; Morris, S.T. Growth Performance and Carcass Characteristics of Lambs Grazing Forage Mixes Inclusive of Plantain (Plantago lanceolata L.) and Chicory (Cichorium intybus L.). Small Rumin. Res. 2015, 127, 20–27. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Garcia, F.; Jurie, C.; Agabriel, J.; Micol, D.; Bauchart, D.; Listrat, A.; Picard, B. Meta-Analysis of the Effect of Animal Maturity on Muscle Characteristics in Different Muscles, Breeds, and Sexes of Cattle. J. Anim. Sci. 2008, 86, 2872–2887. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, P.L.; Harden, S.; Hopkins, D.L. Myofibre Characteristics of Ovine Longissimus and Semitendinosus Muscles Are Influenced by Sire Breed, Gender, Rearing Type, Age, and Carcass Weight. Aust. J. Exp. Agric. 2007, 47, 1137–1146. [Google Scholar] [CrossRef]
- Velleman, S.G.; Coy, C.S.; McFarland, D.C. Effect of Syndecan-1, Syndecan-4, and Glypican-1 on Turkey Muscle Satellite Cell Proliferation, Differentiation, and Responsiveness to Fibroblast Growth Factor 2. Poult. Sci. 2007, 86, 1406–1413. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Canto, I.; Bates, R.O.; Raney, N.E.; Steibel, J.P.; Ernst, C.W. Evaluation of QTL for Carcass Merit and Meat Quality Traits in a US Commercial Duroc Population. Meat Sci. 2012, 92, 132–138. [Google Scholar] [CrossRef]

| Production System | Farm | Sex | Diet 1 | Genetics | Approximate Age at Slaughter (Months) |
|---|---|---|---|---|---|
| REDC-W | A | Wethers | Red Clover | Perendale × LambSupreme 2 | 6–8 |
| MXME-W | B | Wethers | Pasture | Merino | 12 |
| GRASS-W | C | Wethers | Pasture | Composite 3 | 6–8 |
| CHIC-W | C | Wethers | Chicory | Composite 3 | 6–8 |
| CHIC-E | C | Ewes | Chicory | Composite 3 | 6–8 |
| WEAN-W | C | Wethers | Pre-weaning | Composite 3 | 4 |
| Protein | REDC-W | MXME-W | GRASS-W | CHIC-W | CHIC-E | WEAN-W | Accession Number |
|---|---|---|---|---|---|---|---|
| Myofibrillar proteins 1 | |||||||
| Troponin C, skeletal muscle isoform X1 | AB | AB | AB | B | A | A | XP_027832152.1 |
| Actin, alpha skeletal muscle isoform X4 | A | A | A | B | A | A | XP_004021390.1 |
| Myosin-2 | A | A | A | B | A | A | XP_027830685.1 |
| Myosin-8 | A | AB | AB | B | AB | AB | XP_027830687.1 |
| Myosin light chain 1 | AB | A | AB | A | B | AB | A0A0H3V384 |
| Myosin regulatory light chain 2, skeletal muscle isoform | A | A | A | B | A | A | NP_001138655.1 |
| Myosin regulatory light chain 2, skeletal muscle isoform isoform X1 | A | A | A | B | A | A | XP_011959304.1 |
| Sarcoplasmic proteins 1 | |||||||
| Hemoglobin subunit beta | AB | AB | A | AB | B | A | NP_001091117.1 |
| Heat shock protein family A (Hsp70) | A | B | B | B | B | B | W5NPN4 |
| Heat shock cognate 71 kDa protein | A | B | B | B | B | B | XP_011951023.2 |
| Creatine kinase U-type, mitochondrial | B | A | B | B | B | B | XP_011954300.1 |
| Creatine kinase B-type | A | A | AB | B | AB | AB | XP_027813246.1 |
| Immunoglobulin lambda-1 light chain-like | BCD | A | ABC | CD | AB | D | XP_027812629.1 |
| Stromal proteins 1 | |||||||
| Collagen alpha-3(VI) chain | AB | AB | AB | A | B | AB | XP_027823071.1 |
| Collagen type VI alpha 3 chain | AB | AB | AB | A | B | AB | W5QCP9 |
| Proteoglycan 1 | |||||||
| Proteoglycan protein 1 | |||||||
| Heparan sulfate proteoglycan 2 | AB | A | ABC | C | BC | AB | W5PEL7 |
| Protein | Fold Change (Ewe/Wether) | Adjusted p-Values 1 | Coverage (%) | Unique Peptides | Accession |
|---|---|---|---|---|---|
| Myofibrillar proteins | |||||
| Actin, alpha skeletal muscle isoform X4 | 12.88 | <0.001 | 76 | 3 | XP_004021390.1 |
| Myosin-2 | 5.48 | <0.001 | 61 | 3 | XP_027830685.1 |
| Myosin regulatory light chain 2, skeletal muscle isoform | 5.12 | <0.001 | 85 | 6 | NP_001138655.1 |
| Myosin regulatory light chain 2, skeletal muscle isoform isoform X1 | 9.27 | <0.001 | 99 | 3 | XP_011959304.1 |
| Troponin C, skeletal muscle isoform X1 | 2.58 | 0.038 | 80 | 9 | XP_027832152.1 |
| Myosin light chain 1 | 2.61 | 0.005 | 78 | 2 | A0A0H3V384 |
| Sarcoplasmic proteins | |||||
| Immunoglobulin lambda-1 light chain-like | 2.51 | 0.007 | 35 | 2 | XP_027812629.1 |
| Glutathione S-transferase P | 3.07 | 0.003 | 1 | 2 | W5QCP9 |
| Stromal proteins | |||||
| Collagen alpha-3(VI) chain | 0.33 | <0.001 | 1 | 2 | XP_027823071.1 |
| Protein | Folder Change (Grass/Chicory) | Adjusted p-Values 1 | Coverage (%) | Unique Peptides | Accession |
|---|---|---|---|---|---|
| Myofibrillar proteins | |||||
| Actin, alpha skeletal muscle isoform X4 | 17.22 | <0.001 | 76 | 3 | XP_004021390.1 |
| Myosin regulatory light chain 2, skeletal muscle isoform | 5.22 | <0.001 | 85 | 6 | NP_001138655.1 |
| Myosin regulatory light chain 2, skeletal muscle isoform isoform X1 | 6.21 | <0.001 | 99 | 3 | XP_011959304.1 |
| Myosin-2 | 3.70 | <0.001 | 61 | 3 | XP_027830685.1 |
| Protein | Folder Change (Wean/Chicory) | Adjusted p-Values 1 | Coverage (%) | Unique Peptides | Accession |
|---|---|---|---|---|---|
| Myofibrillar proteins | |||||
| Actin alpha skeletal muscle isoform X4 | 16.66 | <0.001 | 76 | 3 | XP_004021390.1 |
| Myosin regulatory light chain 2 skeletal muscle isoform | 6.25 | <0.001 | 85 | 6 | NP_001138655.1 |
| Myosin regulatory light chain 2 skeletal muscle isoform X1 | 5.26 | <0.001 | 99 | 3 | XP_011959304.1 |
| Myosin-2 | 5.26 | <0.001 | 61 | 3 | XP_027830685.1 |
| Myosin-8 | 2.33 | 0.032 | 42 | 7 | XP_027830687.1 |
| Troponin C skeletal muscle isoform X1 | 2.86 | 0.002 | 80 | 9 | XP_027832152.1 |
| Proteoglycan | |||||
| Proteoglycan protein | |||||
| Heparan sulfate proteoglycan 2 | 2.77 | 0.003 | 1 | 2 | W5PEL7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Maes, E.; Deb-Choudhury, S.; Hefer, C.A.; Schreurs, N.M.; Realini, C.E. Proteomic Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems. Foods 2022, 11, 1419. https://doi.org/10.3390/foods11101419
Ye Y, Maes E, Deb-Choudhury S, Hefer CA, Schreurs NM, Realini CE. Proteomic Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems. Foods. 2022; 11(10):1419. https://doi.org/10.3390/foods11101419
Chicago/Turabian StyleYe, Yangfan, Evelyne Maes, Santanu Deb-Choudhury, Charles A. Hefer, Nicola M. Schreurs, and Carolina E. Realini. 2022. "Proteomic Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems" Foods 11, no. 10: 1419. https://doi.org/10.3390/foods11101419
APA StyleYe, Y., Maes, E., Deb-Choudhury, S., Hefer, C. A., Schreurs, N. M., & Realini, C. E. (2022). Proteomic Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems. Foods, 11(10), 1419. https://doi.org/10.3390/foods11101419