Zearalenone Degradation by Dielectric Barrier Discharge Cold Plasma: The Kinetics and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus
2.3. Treatment of Zearalenone with Different Plasma Parameters
2.4. Degradation Efficiency Determination
2.5. Degradation Kinetics of Zearalenone
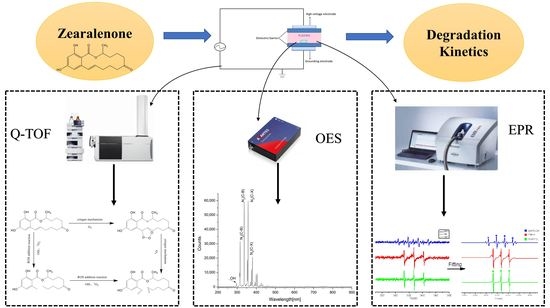
2.6. Structural Elucidation of Degradation Products
2.7. The Active Species Diagnosis by Optical Emission Spectroscopy
2.8. Free Radical Identification by Electron Spin Resonance (ESR)
2.9. Statistical Analysis of Data
3. Results
3.1. Treatment of Zearalenone with Different Plasma Parameters
3.2. Degradation Kinetics of Zearalenone
3.3. Structural Elucidation of Degradation Product
3.4. The Active Species Diagnosis by Optical Emission Spectroscopy
3.5. Free Radical Identification by ESR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, R.; Crisci, A.; Venancio, A.; Abrahantes, J.C.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guine, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—Arevisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Calado, T.; Abrunhosa, L.; Cabo Verde, S.; Alte, L.; Venancio, A.; Fernandez-Cruz, M.L. Effect of gamma-radiation on zearalenone-degradation, cytotoxicity and estrogenicity. Foods 2020, 9, 1687. [Google Scholar] [CrossRef]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic Fungi and Mycotoxins in agricultural crop commodities in the philippines: A review. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Gaumy, J.L.; Bailly, J.D.; Burgat, V.; Guerre, P. Zearalenone: Properties and experimental toxicity. Rev. Med. Vet. 2001, 152, 219–234. [Google Scholar]
- Luo, X.; Zhai, Y.; Qi, L.; Pan, L.; Wang, J.; Xing, J.; Wang, R.; Wang, L.; Zhang, Q.; Yang, K.; et al. Influences of electron beam irradiation on the physical and chemical properties of zearalenone- and ochratoxin a-contaminated corn and in vivo toxicity assessment. Foods 2020, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Agun, L.; Ahmad, N.; Redzuan, N.; Idirs, N.A.S.; Taib, S.M.; Zakaria, Z.; Raja Ibrahim, R.K. Sterilization of oyster mushroom crop residue substrate by using cold plasma technology. Mater. Today Proc. 2020, 39, 903–906. [Google Scholar] [CrossRef]
- Nunes, V.M.R.; Moosavi, M.; Khaneghah, A.M.; Oliveira, C.A.F. Innovative modifications in food processing to reduce the levels of mycotoxins. Curr. Opin. Food Sci. 2021, 38, 155–161. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the role of plasma technology in food industry. Food Bioprocess. Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Shi, H.; Ileleji, K.; Stroshine, R.L.; Keener, K.; Jensen, J.L. Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioprocess. Technol. 2017, 10, 1042–1052. [Google Scholar] [CrossRef]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A.d.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- GB 5009.209-2016, 16; The National Standard for Food Safety, Determination of Zearalenone in Food of the People’s Republic of China. National Health and Family Planning Commission: Beijing, China, 2016.
- Siciliano, I.; Spadaro, D.; Prelle, A.; Vallauri, D.; Cavallero, M.C.; Garibaldi, A.; Gullino, M.L. Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins 2016, 8, 125. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Puligundla, P.; Lee, T.; Mok, C. Effect of corona discharge plasma jet treatment on the degradation of aflatoxin B-1 on glass slides and in spiked food commodities. LWT 2020, 124, 108333. [Google Scholar] [CrossRef]
- Wang, S.Q.; Huang, G.Q.; Li, Y.P.; Xiao, J.X.; Zhang, Y.; Jiang, W.L. Degradation of aflatoxin B-1 by low-temperature radio frequency plasma and degradation product elucidation. Eur. Food Res. Technol. 2015, 241, 103–113. [Google Scholar] [CrossRef]
- Schaffner, D.W.; Labuza, T.P. Predictive microbiology: Where are we, and where are we going? Food Technol. 1997, 51, 95–99. [Google Scholar]
- ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viol, W.; Karlovsky, P. Plasma-based degradation of mycotoxins produced by fusarium, aspergillus and alternaria species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.H.; Wang, R.; Wang, L.; Wang, Y.; Chen, Z.X. Structure elucidation and toxicity analyses of the degradation products of aflatoxin B-1 by aqueous ozone. Food Control 2013, 31, 331–336. [Google Scholar] [CrossRef]
- Luo, X.H.; Li, K.; Xing, J.L.; Qi, L.J.; Yang, M.; Wang, R.; Wang, L.; Li, Y.A.; Chen, Z.X. In vivo toxicity assessment of aflatoxin B-1-contaminated corn after ozone degradation. Food Addit. Contam. Part A 2018, 35, 341–350. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.N.; Zhang, Q.; Feng, H.Q.; Liang, Y.D.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.N.; Milosavljevic, V.; Bourke, P.; O’Regan, F.; Cullen, P.J. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process. Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Mosnier, J.-P.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of cherry tomatoes. J. Biosci. Bioeng. 2014, 118, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.N.; Wang, G.M.; Tian, Y.; Wang, K.L.; Zhang, J.E.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Wang, R.X.; Nian, W.F.; Wu, H.Y.; Feng, H.Q.; Zhang, K.; Zhang, J.; Zhu, W.D.; Becker, K.H.; Fang, J. Atmospheric-pressure cold plasma treatment of contaminated fresh fruit and vegetable slices: Inactivation and physiochemical properties evaluation. Eur. Phys. J. D 2012, 66, 276. [Google Scholar] [CrossRef]
- Wu, H.; Sun, P.; Feng, H.; Zhou, H.; Wang, R.; Liang, Y.; Lu, J.; Zhu, W.; Zhang, J.; Fang, J. Reactive oxygen species in a non-thermal Plasma Microjet and Water System: Generation, Conversion, and Contributions to Bacteria Inactivation-An Analysis by electron spin resonance spectroscopy. Plasma Process. Polym. 2012, 9, 417–424. [Google Scholar] [CrossRef]
- Zhang, B.; Li, R.; Yan, J. Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment. Arch. Biochem. Biophys. 2018, 655, 37–42. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, W.L.; Guo, H.S.; Ren, X.H.; Xu, Q. Cu (II)-doped V2O5 mediated persulfate activation for heterogeneous catalytic degradation of benzotriazole in aqueous solution. Sep. Purif. Technol. 2020, 230, 115848. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Dai, D.J.; Yao, Y.Y.; Chen, L.K.; Liu, Q.B.; Luo, L.S. Extremely enhanced generation of reactive oxygen species for oxidation of pollutants from peroxymonosulfate induced by a supported copper oxide catalyst. Chem. Eng. J. 2017, 322, 546–555. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.M.; Bai, Y.H.; Li, X.J.; Chen, J.R. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Sep. Purif. Technol. 2013, 120, 191–197. [Google Scholar] [CrossRef]











| Model | Equation | F | R2 | Sig. |
|---|---|---|---|---|
| Log function | Y = 32.204 logx − 56.677 | 242.414 | 0.956 | <0.001 |
| Quadratic function | Y = 4.904 + 1.737x − 0.009x2 | 80.380 | 0.935 | <0.001 |
| Cubic function | Y = 2.715x − 0.027 x2 + 9.274e−5x3 − 7.755 | 66.893 | 0.947 | <0.001 |
| Exponential function (S) | 812.195 | 0.987 | <0.001 |
| Compound | Formula | Observed m/z | Retention Time (min) | Mass Error (ppm) |
|---|---|---|---|---|
| Zearalenone | C18H22O5 | 317.1390 | 7.450 | 1.41 |
| A | C18H22O7 | 349.1290 | 5.839 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Huang, Y.; Liu, L.; Chen, Y.; Wang, Y.; Li, C. Zearalenone Degradation by Dielectric Barrier Discharge Cold Plasma: The Kinetics and Mechanism. Foods 2022, 11, 1494. https://doi.org/10.3390/foods11101494
Zheng Z, Huang Y, Liu L, Chen Y, Wang Y, Li C. Zearalenone Degradation by Dielectric Barrier Discharge Cold Plasma: The Kinetics and Mechanism. Foods. 2022; 11(10):1494. https://doi.org/10.3390/foods11101494
Chicago/Turabian StyleZheng, Zhe, Yousheng Huang, Liping Liu, Yi Chen, Yuanxing Wang, and Chang Li. 2022. "Zearalenone Degradation by Dielectric Barrier Discharge Cold Plasma: The Kinetics and Mechanism" Foods 11, no. 10: 1494. https://doi.org/10.3390/foods11101494
APA StyleZheng, Z., Huang, Y., Liu, L., Chen, Y., Wang, Y., & Li, C. (2022). Zearalenone Degradation by Dielectric Barrier Discharge Cold Plasma: The Kinetics and Mechanism. Foods, 11(10), 1494. https://doi.org/10.3390/foods11101494