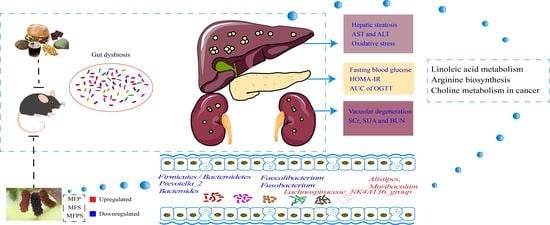
Polyphenols and Polysaccharides from Morus alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction, Isolation, and Purification of Morus alba L. Fruit Polyphenols and Polysaccharides
2.2. Compositional Identification of MFP and MFS
2.3. Animal Treatment
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Sample Collection
2.6. Biochemistry Assays and Histological Examination
2.7. Histological Analysis
2.8. Gut Microbiota Analysis by 16S rDNA Gene Sequencing
2.9. Untargeted Fecal Metabolomics Analysis
2.10. Statistical Analyses
3. Results
3.1. Identification Analysis of MFP and MFS
3.2. Alleviation of Obesity and Fat Accumulation in HFD-Fed C57BL/6J Mice after MFP, MFS and MFPS Treatments
3.3. Prevention of HFD-Induced Hepatic Fat Deposition and Oxidative Stress after MFP, MFS and MFPS Treatments
3.4. Improvement in Glucose Metabolism Disorder after MFP, MFS and MFPS Treatments
3.5. Prevention of Dyslipidemia after MFP, MFS and MFPS Treatments in HFD-Fed Mice
3.6. Alleviation of Renal Injury Phenotypes in HFD-Fed Mice after MFP, MFS and MFPS Treatments
3.7. Effects of MFP, MFS and MFPS on Inflammatory Mediators and Colonic Lesion Phenotypes in HFD-Fed Mice
3.8. Improvement of Gut Microbiota Dysbiosis in HFD-Fed Mice after MFP, MFS and MFPS Treatments
3.9. Changes inf Fecal Metabolites in HFD-Fed Mice after MFP, MFS and MFPS Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mccracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Hu, Y.; Zhang, B.; Shao, Z.; Roura, E.; Wang, S. Tea polyphenol-gut microbiota interactions: Hints on improving the metabolic syndrome in a multi-element and multi-target manner. Food Sci. Hum. Wellness 2022, 11, 11–21. [Google Scholar] [CrossRef]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.L.; Al-Khalidi, H.R.; Friedman, D.J.; Mulder, H.; Kucharska-Newton, A.; Rosamond, W.R.; Lopes, R.D.; Gersh, B.J.; Mark, D.B.; Curtis, L.H.; et al. The metabolic syndrome and risk of sudden cardiac death: The atherosclerosis risk in communities study. J. Am. Heart Assoc. 2017, 6, e006103. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clement, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 2016, 12, 169–181. [Google Scholar] [CrossRef]
- Villard, A.; Boursier, J.; Andriantsitohaina, R. Microbiota-derived extracellular vesicles and metabolic syndrome. Acta Physiol. 2021, 231, e13600. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Zou, X.; Ye, C.; Xia, H.; Yang, M.; Gao, Q.; Yang, Q.; Liu, H. Ligustrum robustum (Roxb.) blume extract modulates gut microbiota and prevents metabolic syndrome in high-fat diet-fed mice. J. Ethnopharmacol. 2021, 268, 113695. [Google Scholar] [CrossRef]
- Chang, B.; Koo, B.; Kim, S. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, S.; Liao, Y.; Ma, C.; Wang, D.; Tong, J.; Feng, J.; Yi, T.; Zhu, L. A systematic review of the medicinal potential of mulberry in treating diabetes mellitus. Am. J. Chin. Med. 2019, 46, 1743–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China, 2020 ed.; Chinese Medical Science Press: Beijing, China, 2020; p. 313. [Google Scholar]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimaraes, R.; Freitas, K.C. Nutraceutical and medicinal potential of the morus species in metabolic dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Shuang, F.; Fu, Q.; Ju, Y.; Zong, C.; Zhao, W.; Zhang, D.; Yao, X.; Cao, F. Evaluation of the Chemical Composition and Antioxidant Activity of Mulberry (Morus alba L.) Fruits from Different Varieties in China. Molecules 2022, 27, 2688. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, Z.; Chen, C.; Lu, B.; Liu, D. Targeting gut microbiota with natural polysaccharides: Effective interventions against High-Fat Diet-Induced metabolic diseases. Front. Microbiol. 2022, 13, 859206. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef]
- Long, X.S.; Liao, S.T.; Wen, P.; Zou, Y.X.; Liu, F.; Shen, W.Z.; Hu, T.G. Superior hypoglycemic activity of mulberry lacking monosaccharides is accompanied by better activation of the PI3K/Akt and AMPK signaling pathways. Food Funct. 2020, 11, 4249–4258. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Li, X.; Zhu, C.; Sun, J.; Tian, L.; Chen, W.; Bai, W. The target cells of anthocyanins in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2019, 59, 921–946. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wen, P.; Fu, H.; Lin, G.; Liao, S.; Zou, Y. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 1, 1513–1528. [Google Scholar] [CrossRef]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Antimetabolic syndrome effect of phytosome containing the combined extracts of mulberry and ginger in an animal model of metabolic syndrome. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Qiu, F.; Zhao, H.; Tang, H.; Li, X.; Shi, D. Dietary flaxseed oil prevents Western-Type Diet-Induced nonalcoholic fatty liver disease in Apolipoprotein-E knockout mice. Oxid. Med. Cell. Longev. 2017, 2017, 3256241. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Lai, Y.; Huang, H.; Lee, I.; Lin, L.; Liu, H.; Tien, H.; Huang, C. Ginseng-plus-Bai-Hu-Tang ameliorates diet-induced obesity, hepatic steatosis, and insulin resistance in mice. J. Ginseng. Res. 2020, 44, 238–246. [Google Scholar] [CrossRef]
- Watanabe, H.; Obata, H.; Watanabe, T.; Sasaki, S.; Nagai, K.; Aizawa, Y. Metabolic syndrome and risk of development of chronic kidney disease: The Niigata preventive medicine study. Diabetes/Metab. Res. Rev. 2010, 26, 26–32. [Google Scholar] [CrossRef]
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological potential of polyphenols in the context of metabolic syndrome: An analysis of studies on animal models. Biology 2022, 11, 559. [Google Scholar] [CrossRef]
- Hu, B.; Ye, C.; Leung, E.L.; Zhu, L.; Hu, H.; Zhang, Z.; Zheng, J.; Liu, H. Bletilla striata oligosaccharides improve metabolic syndrome through modulation of gut microbiota and intestinal metabolites in high fat diet-fed mice. Pharmacol. Res. 2020, 159, 104942. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, F.; Hu, H.; Li, X.; Dong, F.; Sun, M.; Xiao, Y.; Ge, T.; et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut. Microbes. 2021, 13, 1865708. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses—A review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Chan, A.M.L.; Ng, A.M.H.; Mohd Yunus, M.H.; Idrus, R.B.H.; Law, J.X.; Yazid, M.D.; Chin, K.; Shamsuddin, S.A.; Lokanathan, Y. Recent developments in rodent models of High-Fructose Diet-Induced metabolic syndrome: A systematic review. Nutrients 2021, 13, 2497. [Google Scholar] [CrossRef]
- Wainwright, P.; Byrne, C. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef] [Green Version]
- Declèves, A.; Mathew, A.V.; Armando, A.M.; Han, X.; Dennis, E.A.; Quehenberger, O.; Sharma, K. AMP-activated protein kinase activation ameliorates eicosanoid dysregulation in high-fat-induced kidney disease in mice. J. Lipid. Res. 2019, 60, 937–952. [Google Scholar] [CrossRef] [Green Version]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Seshan, S.V.; Cohen-Gould, L.; Manichev, V.; Feldman, L.C.; Gustafsson, T. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1beta and IL-18 and arrests CKD. J. Am. Soc. Nephrol. 2017, 28, 1437–1449. [Google Scholar] [CrossRef] [Green Version]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fan, X.; Ye, R.; Hu, Y.; Zheng, T.; Shi, R.; Cheng, W.; Lv, X.; Chen, L.; Liang, P. The effect of simvastatin on gut microbiota and lipid metabolism in hyperlipidemic rats induced by a High-Fat diet. Front. Pharmacol. 2020, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfora, E.E.; Meex, R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6r–14r. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, S.; D Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary strategies for management of metabolic syndrome: Role of gut microbiota metabolites. Nutrients 2021, 13, 1389. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Ijaz-Ul-Haq; Chen, A.; Xu, C.; Jianglei, R.; Feng, Q.; Li, M. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.; Lee, K.; Sung, J.; Ko, G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 2017, 66, 1031–1038. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, L.; Wan, Y.; Liu, C.; Jiang, S.; Shang, E.; Duan, J. Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats. J. Chromatogr. B 2021, 1174, 122728. [Google Scholar] [CrossRef]
- Huang, S.; Pang, D.; Li, X.; You, L.; Zhao, Z.; Cheung, P.C.; Zhang, M.; Liu, D. A sulfated polysaccharide from Gracilaria Lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct. 2019, 10, 3224–3236. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, H.B.; Lee, E.; Park, H.Y. The effects of gelatinized wheat starch and high salt diet on gut microbiota and metabolic disorder. Nutrients 2020, 12, 301. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D.; Cui, J.Y. Review: Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab. Dispos. 2015, 43, 1505–1521. [Google Scholar] [CrossRef] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafra, D.; Lobo, J.C.; Barros, A.F.; Koppe, L.; Vaziri, N.D.; Fouque, D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014, 9, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Yang, C.; Shi, Z.; Wang, Z.; Jiang, D. Analysis of serum metabolomics in obese mice induced by High-Fat diet. Diabetes Metab. Syndr. Obes. 2021, 14, 4671–4678. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Poloncová, K.; Griač, P. Phospholipid transport and remodeling in health and disease. Gen. Physiol. Biophys. 2011, 30, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehedint, M.G.; Zeisel, S.H. Choline’s role in maintaining liver function: New evidence for epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, Q.; Xia, T.; Fu, S.; Tao, X.; Wen, Y.; Chan, S.; Gao, S.; Xiong, X.; Chen, W. Diagnostic value of plasma tryptophan and symmetric dimethylarginine levels for acute kidney injury among tacrolimus-treated kidney transplant patients by targeted metabolomics analysis. Sci. Rep. 2018, 8, 14688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, A.; Ikeda, M. Amino Acid Fermentation; Springer: Tokyo, Japan, 2017. [Google Scholar]
- Xie, G.; Ma, X.; Zhao, A.; Wang, C.; Zhang, Y.; Nieman, D.; Nicholson, J.K.; Jia, W.; Bao, Y.; Jia, W. The metabolite profiles of the obese population are Gender-Dependent. J. Proteome Res. 2014, 13, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract. 2015, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bohler, M.; van den Berg, E.H.; Almanza, M.; Connelly, M.A.; Bakker, S.; de Meijer, V.E.; Dullaart, R.; Blokzijl, H. Branched chain amino acids are associated with metabolic complications in liver transplant recipients. Clin. Biochem. 2022, 102, 26–33. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Li, Q.; Lei, Q.; Zhou, D.; Wang, S. Polyphenols and Polysaccharides from Morus alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile. Foods 2022, 11, 1818. https://doi.org/10.3390/foods11121818
Wan M, Li Q, Lei Q, Zhou D, Wang S. Polyphenols and Polysaccharides from Morus alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile. Foods. 2022; 11(12):1818. https://doi.org/10.3390/foods11121818
Chicago/Turabian StyleWan, Meixia, Qing Li, Qianya Lei, Dan Zhou, and Shu Wang. 2022. "Polyphenols and Polysaccharides from Morus alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile" Foods 11, no. 12: 1818. https://doi.org/10.3390/foods11121818
APA StyleWan, M., Li, Q., Lei, Q., Zhou, D., & Wang, S. (2022). Polyphenols and Polysaccharides from Morus alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile. Foods, 11(12), 1818. https://doi.org/10.3390/foods11121818