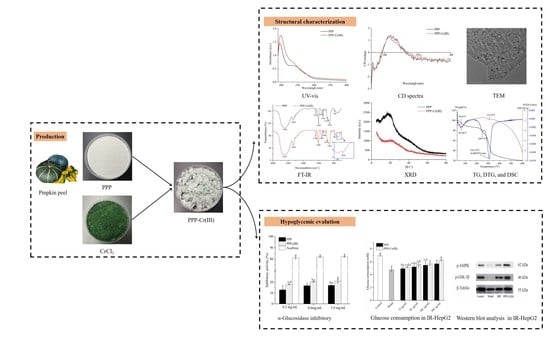
Structural Characterization and Hypoglycemic Activity of a Novel Pumpkin Peel Polysaccharide-Chromium(III) Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Purification of PPP
2.3. Synthesis of PPP-Cr(III) Complex
2.4. Physicochemical Properties of PPP-Cr(III) Complex
2.5. The Content of Cr Element
2.6. Characterization of PPP-Cr(III) Complex
2.6.1. Molecular Weight Analysis
2.6.2. Monosaccharide Composition Analysis
2.6.3. UV-Vis and FT-IR Spectra Analysis
2.6.4. CD Spectra Analysis
2.6.5. XRD Analysis
2.6.6. Thermal Analysis
2.7. Hypoglycemic Activity
2.7.1. α-Glucosidase Inhibitory Activity
2.7.2. Hypoglycemic Activity in HepG2 Cells Model
2.7.2.1. Cell Culture of HepG2 Cells
2.7.2.2. HepG2 Cells Viability Assay
2.7.2.3. Evaluation of the Glucose Consumption Capacity in Insulin Resistance (IR)-Hep G2 Cells
2.7.2.4. Western Blot Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Monosaccharide Composition
3.3. Molecular Weight
3.4. UV-Vis and FT-IR Spectra Analysis
3.5. CD Spectra
3.6. XRD Pattern Analysis
3.7. Thermal Analysis
3.8. Hypoglycemic Activity
3.8.1. α-Glucosidase Inhibitory Activity
3.8.2. Cell Viability
3.8.3. Glucose Consumption in IR-HepG2 Cells
3.8.4. PPP and PPP-Cr(III) Stimulated p-AMPK/p-GSK-3β Express in IR-HepG2 Cell Culture
3.9. Structure-Hypoglycemic Activity of PPP-Cr(III)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lu, A.; Yu, M.; Fang, Z.; Xiao, B.; Guo, L.; Wang, W.; Li, J.; Wang, S.; Zhang, Y. Preparation of the controlled acid hydrolysates from pumpkin polysaccharides and their antioxidant and antidiabetic evaluation. Int. J. Biol. Macromol. 2019, 121, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Qian, L.; Yin, D.L.; Zhou, Y. Hypolipidemic effect of the polysaccharides extracted from pumpkin by cellulase-assisted method on mice. Int. J. Biol. Macromol. 2014, 64, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746. [Google Scholar] [CrossRef]
- Dhenge, R.; Rinaldi, M.; Ganino, T.; Santi, S.; Ferrarese, I.; Dall’Acqua, S. Variations of polyphenols, sugars, carotenoids, and volatile constituents in pumpkin (Cucurbita moschata) during high pressure processing: A kinetic study. Innov. Food Sci. Emerg. Technol. 2022, 78, 103005. [Google Scholar] [CrossRef]
- Zhou, T.; Kong, Q.; Huang, J.; Dai, R.; Li, Q. Characterization of nutritional compounds and utilization of pumpkin. Food 2007, 1, 313–321. [Google Scholar]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Perez-Cordoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef]
- Jun, H.I.; Lee, C.H.; Song, G.S.; Kim, Y.S. Characterization of the pectic polysaccharides from pumpkin peel. LWT Food Sci. Technol. 2006, 39, 554–561. [Google Scholar] [CrossRef]
- Bai, D.S.; Ma, D.; Rui, Q.; Zhang, J. [Extraction, purification and properties of pumpkin peel polysaccharide PP1]. Food Sci. 2012, 33, 122–126. (In Chinese) [Google Scholar]
- Wang, Q.; Li, G.J.; Zhou, Y.L.; Zhao, X. [Chemical composition and antioxidant activity of crude polysaccharides extracted from pumpkin peel as affected by different extraction methods]. Food Sci. 2013, 34, 118–121. (In Chinese) [Google Scholar] [CrossRef]
- Dey, M.; Das, M.; Chowhan, A.; Giri, T.K. Breaking the barricade of oral chemotherapy through polysaccharide nanocarrier. Int. J. Biol. Macromol. 2019, 130, 34–49. [Google Scholar] [CrossRef]
- Gao, P.; Bian, J.; Xu, S.; Liu, C.; Sun, Y.; Zhang, G.; Li, D.; Liu, X. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef]
- Jia, Y.; Li, N.; Wang, Q.; Zhou, J.; Liu, J.; Zhang, M.; He, C.; Chen, H. Effect of Fe (III), Zn (II), and Cr (III) complexation on the physicochemical properties and bioactivities of corn silk polysaccharide. Int. J. Biol. Macromol. 2021, 189, 847–856. [Google Scholar] [CrossRef]
- Ognik, K.; Dworzanski, W.; Sembratowicz, I.; Fotschki, B.; Cholewinska, E.; Listos, P.; Juskiewicz, J. The effect of the high-fat diet supplemented with various forms of chromium on rats body composition, liver metabolism and organ histology Cr in liver metabolism and histology of selected organs. J. Trace Elem. Med. Biol. 2021, 64, 126705. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Zhu, T.; Zhang, X.; Jin, M.; Jiao, L.; Meng, F.; Figueiredo-Silva, C.; Hong, Y.; Zhou, Q. Dietary chromium could improve growth, antioxidant capacity, chromium accumulation in tissues and expression of genes involved into glucose and lipid metabolism in juvenile mud crab Scylla paramamosain. Aquacult. Rep. 2022, 23, 101088. [Google Scholar] [CrossRef]
- Wang, X.; Ye, H.; Cui, J.; Chi, Y.; Liu, R.; Wang, P. Hypolipidemic effect of chromium-modified enzymatic product of sulfated rhamnose polysaccharide from Enteromorpha prolifera in type 2 diabetic mice. Marine Life Sci. Technol. 2022, 4, 245–254. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, M.; Pan, W.L.; Zhang, Q.; Xu, J.X.; Lin, Y.C.; Li, L.; Liu, B.; Bai, W.D.; Zhang, Y.Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, M.; Hong, R.; Chen, H. Preparation of a Momordica charantia L. polysaccharide chromium (III) complex and its anti-hyperglycemic activity in mice with streptozotocin-induced diabetes. Int. J. Biol. Macromol. 2019, 122, 619–627. [Google Scholar] [CrossRef]
- Li, L.Y.; Qiu, Z.C.; Dong, H.J.; Ma, C.X.; Qiao, Y.T.; Zheng, Z.J. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: A comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.X.; Cao, Y.J.; Lin, Y.C.; Guo, W.L.; Liu, J.Y.; Bai, W.D.; Zhang, Y.Y.; Ni, L.; Liu, B.; et al. Preparation of Ganoderma lucidum polysaccharidechromium (III) complex and its hypoglycemic and hypolipidemic activities in high-fat and high-fructose diet-induced pre-diabetic mice. Int. J. Biol. Macromol. 2019, 140, 782–793. [Google Scholar] [CrossRef]
- Saha, S.K.; Brewer, C.F. Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydr. Res. 1994, 254, 157–167. [Google Scholar] [CrossRef]
- Bradord, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guo, W.L.; Shi, F.F.; Li, L.; Xu, J.X.; Chen, M.; Wu, L.; Hong, J.L.; Qian, M.; Bai, W.D.; Liu, B.; et al. Preparation of a novel Grifola frondosa polysaccharide-chromium (III) complex and its hypoglycemic and hypolipidemic activities in high fat diet and streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 131, 81–88. [Google Scholar] [CrossRef]
- Yang, Y.M.; Qiu, Z.C.; Li, L.Y.; Vidyarthi, S.K.; Zheng, Z.J.; Zhang, R.T. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef]
- Lu, Y.L.; Liang, J.; Zhou, F.Y.; Kuang, H.X.; Xia, Y.G. Discrimination and characterization of Panax polysaccharides by 2D COS-IR spectroscopy with chemometrics. Int. J. Biol. Macromol. 2021, 183, 193–202. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wu, X.F.; Zhang, K.; Sun, F.J.; Zhou, W.L.; Wu, Z.Q.; Li, X.T. Purification, characterization, and emulsification stability of high- and low-molecular-weight fractions of polysaccharide conjugates extracted from green tea. Food Hydrocolloids. 2022, 129, 107667. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.J.; Chen, S.W.; Xia, Y.M.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on alpha-amylase and alpha-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Wu, J.S.; Huang, R.; Jiao, D.X.; Liu, S.Y.; Liu, H.M.; Liu, H.Z. Protection by Hosta ventricosa polysaccharides against oxidative damage induced by t-BHP in HepG2 cells via the JNK/Nrf2 pathway. Int. J. Biol. Macromol. 2022, 208, 453–462. [Google Scholar] [CrossRef]
- Wang, Z.C.; Sun, L.L.; Fang, Z.X.; Nisar, T.; Zou, L.; Li, D.; Guo, Y.R. Lycium ruthenicum Murray anthocyanins effectively inhibit α-glucosidase activity and alleviate insulin resistance. Food Biosci. 2021, 41, 100949. [Google Scholar] [CrossRef]
- Dong, F.; Zheng, H.Z.; Jeong, W.S.; Chung, S.K.; Qu, Z.Y.; Zou, X.; Liu, C.; Xiang, Q.; Feng, F. Synthesis, characterization, and antioxidant activity in vitro of selenium-Euryale ferox Salisb. polysaccharide. Appl. Biol. Chem. 2021, 64, 59. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhao, B.T.; Wang, X.F.; Yao, J.; Zhang, J. Synthesis of selenium-containing polysaccharides and evaluation of antioxidant activity in vitro. Int. J. Biol. Macromol. 2012, 51, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, J.; Wei, Y.L.; Jiao, X.; Li, Q.H. Holistic review of polysaccharides isolated from pumpkin: Preparation methods, structures and bioactivities. Int. J. Biol. Macromol. 2021, 193, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, H.; Shen, Y.; Wang, Y.L.; Zhao, Z.M.; Zhang, Y. Preparation, characterization and antioxidant activity evaluation in vitro of Fritillaria ussuriensis polysaccharide-zinc complex. Int. J. Biol. Macromol. 2020, 146, 462–474. [Google Scholar] [CrossRef]
- Rao, C.P.; Kaiwar, S.P. Chromate reduction: Reduction of potassium chromate by D-glucose and D-fructose to form Cr(III)-saccharide complexes. Carbohydr. Res. 1992, 237, 195–202. [Google Scholar] [CrossRef]
- Geetha, K.; Raghavan, M.S.S.; Kulshreshtha, S.K.; Sasikala, R.; Rao, C.P. Transition-metal saccharide chemistry synthesis, spectroscopy, electrochemistry and magnetic susceptibility studies of iron(Ill)complexes of mono- and disaccharides. Carbohydr. Res. 1995, 271, 163–175. [Google Scholar] [CrossRef]
- Yuan, L.L.; Qiu, Z.C.; Yang, Y.M.; Liu, C.; Zhang, R.T. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 2022, 385, 132637. [Google Scholar] [CrossRef]
- Ranjbar, B.; Gill, P. Circular dichroism techniques: Biomolecular and nanostructural analyses—A review. Chem. Biol. Drug. Des. 2009, 74, 101–120. [Google Scholar] [CrossRef]
- Park, J.W.; Chakrabarti, B. Optical characteristic of carboxyl group in relation to the circular dichroic properties and dissociation constants of glycosaminoglycans. Biochim. Biophys. Acta. 1978, 544, 667–675. [Google Scholar] [CrossRef]
- Zhao, M.M.; Bai, J.W.; Bu, X.Y.; Yin, Y.T.; Wang, L.B.; Yang, Y.; Xu, Y.Q. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on alpha-amylase and alpha-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.Q.; Pan, Y.X.; Gao, X.D.; Chen, H.X. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Xiong, G.Y.; Ma, L.S.; Zhang, H.; Li, Y.P.; Zou, W.S.; Wang, X.F.; Xu, Q.S.; Xiong, J.T.; Hu, Y.P.; Wang, X.Y. Physicochemical properties, antioxidant activities and alpha-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022, 194, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Ren, L.; Liu, W.J.; Sui, Y.; Nong, Q.N.; Xiao, Q.H.; Li, X.Q.; Cao, W. Structural characteristics of a hypoglycemic polysaccharide from Fructus Corni. Carbohydr. Res. 2021, 506, 108358. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Gao, J.; Du, M.; Mao, X.Y. Casein glycomacropeptide hydrolysates ameliorate hepatic insulin resistance of C57BL/6J mice challenged with high-fat diet. J. Funct. Foods. 2018, 45, 190–198. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.P.; Cui, J.F.; Zhu, Y.J.; Li, Y.Y.; Chi, Y.Z.; Wang, J.F.; Wang, P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorg. Chem. 2019, 88, 102942. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.J.; Fan, X.Y.; Qian, Y.W.; Lv, Q.Y.; Teng, H. Sonchus oleraceus Linn extract enhanced glucose homeostasis through the AMPK/Akt/GSK-3β signaling pathway in diabetic liver and HepG2 cell culture. Food Chem. Toxicol. 2020, 136, 111072. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Cui, W.; Eskin, N.A.M.; Goff, H.D. A molecular modeling approach to understand conformation–functionality relationships of galactomannans with different mannose/galactose ratios. Food Hydrocolloids 2012, 26, 359–364. [Google Scholar] [CrossRef]
- Chen, S.; Khan, B.M.; Cheong, K.L.; Liu, Y. Pumpkin polysaccharides: Purification, characterization and hypoglycemic potential. Int. J. Biol. Macromol. 2019, 139, 842–849. [Google Scholar] [CrossRef]
- Deng, Y.J.; Huang, L.X.; Zhang, C.H.; Xie, P.J.; Cheng, J.; Wang, X.; Liu, L.J. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Food Biosci. 2020, 153, 755–766. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Guo, Y.Y.; Duan, S.Y.; Wei, H.; Liu, Y.S.; Wang, L.B.; Huo, X.; Yang, Y. Effects of ultrasound irradiation on the characterization and bioactivities of the polysaccharide from blackcurrant fruits. Ultrason. Sonochem. 2018, 49, 206–214. [Google Scholar] [CrossRef]
- Hoffman, N.J.; Penque, B.A.; Habegger, K.M.; Sealls, W.; Tackett, L.; Elmendorf, J.S. Chromium enhances insulin responsiveness via AMPK. J. Nutr. Biochem. 2014, 25, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Zhong, J.; Long, J.; Zou, X.Y.; Wang, D.; Song, Y.; Zhou, K.; Liang, Y.X.; Huang, R.M.; Wei, X.Q.; et al. Hypoglycemic effects and mechanism of different molecular weights of konjac glucomannans in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 165, 2231–2243. [Google Scholar] [CrossRef] [PubMed]




| Samples | PPP | PPP-Cr(III) |
|---|---|---|
| Total saccharides content (%) | 77.79 ± 4.18 | 61.16 ± 1.17 |
| Protein content (%) | 1.18 ± 0.05 | 1.13 ± 0.05 |
| Chromium content (mg/g) | - | 23.77 ± 0.39 |
| Monosaccharides composition (molar ratio, %) | ||
| Rhamnose | 0.52 | 0.27 |
| Arabinose | 2.24 | 1.72 |
| Galactose | 4.81 | 3.53 |
| Glucose | 86.24 | 89.63 |
| Galacturonic acid | 6.20 | 4.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, L.; Ma, Y.; Chen, X.; Lan, T.; Chen, L.; Zheng, Z. Structural Characterization and Hypoglycemic Activity of a Novel Pumpkin Peel Polysaccharide-Chromium(III) Complex. Foods 2022, 11, 1821. https://doi.org/10.3390/foods11131821
Zhang W, Li L, Ma Y, Chen X, Lan T, Chen L, Zheng Z. Structural Characterization and Hypoglycemic Activity of a Novel Pumpkin Peel Polysaccharide-Chromium(III) Complex. Foods. 2022; 11(13):1821. https://doi.org/10.3390/foods11131821
Chicago/Turabian StyleZhang, Wen, Lingyu Li, Yue Ma, Xiaole Chen, Tao Lan, Long Chen, and Zhenjia Zheng. 2022. "Structural Characterization and Hypoglycemic Activity of a Novel Pumpkin Peel Polysaccharide-Chromium(III) Complex" Foods 11, no. 13: 1821. https://doi.org/10.3390/foods11131821
APA StyleZhang, W., Li, L., Ma, Y., Chen, X., Lan, T., Chen, L., & Zheng, Z. (2022). Structural Characterization and Hypoglycemic Activity of a Novel Pumpkin Peel Polysaccharide-Chromium(III) Complex. Foods, 11(13), 1821. https://doi.org/10.3390/foods11131821