Alleviation of Metabolic Disturbance by Substituting Kanjang High in Bacillus for Salt through Modulation of Gut Microbiota in Estrogen-Deficient Rats
Abstract
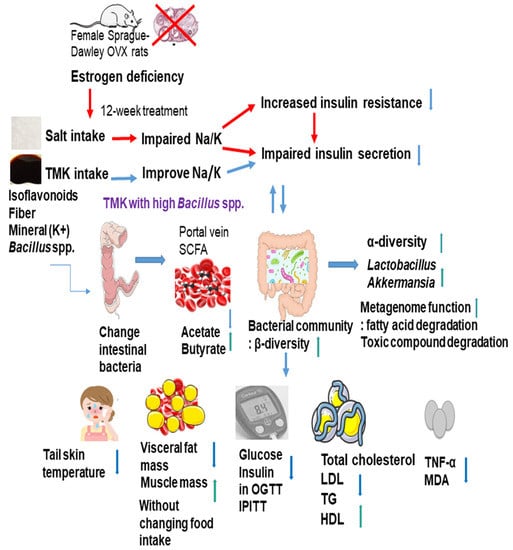
:1. Introduction
2. Materials and Methods
2.1. General Production Process of TMK and Its Collection
2.2. Ovariectomy Operation
2.3. Diet Preparation
2.4. Experimental Design
2.5. Tail Skin Temperature
2.6. Body Composition
2.7. Glucose Homeostasis and Sample Collection
2.8. Islet Isolation, Total RNA Generation, and Gene Expression by Real-Time PCR
2.9. Serum SCFA Concentrations and Gut Microbiome by Next-Generation Sequencing (NGS)
2.10. Metagenome Functions of the Fecal Bacteria by PICRUSt2 Pipeline Analysis
2.11. Statistical Analysis
3. Results
3.1. General Characteristics of TMK with Different Levels of Bacillus and Biogenic Amines
3.2. Serum 17β-Estradiol Concentration, Uterine Weight, and Tail Skin Temperature
3.3. Energy Metabolism and Body Composition
3.4. Glucose Metabolism
3.5. Lipid Profiles
3.6. Liver Metabolism
3.7. SCFA Concentration from the Portal Vein Blood
3.8. Gut Microbiota Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, A.C.; Silva, A.M.; Santos, M.S.; Sardao, V.A. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid. Biochem. Mol. Biol. 2014, 143, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Ryuk, J.A.; Park, S. Supplementing with Opuntia ficus-indica Mill and Dioscorea nipponica Makino extracts synergistically attenuates menopausal symptoms in estrogen-deficient rats. J. Ethnopharmacol. 2014, 155, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Lee, Y.H.; Park, S. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp. Biol. Med. 2017, 242, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Kahleova, H.; Holtz, D.N.; del Aguila, F.; Neola, M.; Crosby, L.M.; Holubkov, R. The Women’s Study for the Alleviation of Vasomotor Symptoms (WAVS): A randomized, controlled trial of a plant-based diet and whole soybeans for postmenopausal women. Menopause 2021, 28, 1150–1156. [Google Scholar] [CrossRef]
- Purzand, B.; Rokhgireh, S.; Shabani Zanjani, M.; Eshraghi, N.; Mohamadianamiri, M.; Esmailzadeh, A.; Alkatout, I.; Gitas, G.; Allahqoli, L. The comparison of the effect of soybean and fish oil on supplementation on menopausal symptoms in postmenopausal women: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Clin. Pract. 2020, 41, 101239. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Impact of Soy Foods on the Development of Breast Cancer and the Prognosis of Breast Cancer Patients. Complementary Med. Res. 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose–response meta-analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.; Lee, D.S.; Suh, H.J.; Park, Y. Aspergillus oryzae fermented germinated soybean extract alleviates perimenopausal symptoms in ovariectomized rats. J. Sci. Food Agric. 2016, 96, 979–987. [Google Scholar] [CrossRef]
- Daily, J.W.; Ko, B.S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, B.; Shu, F.R.; Chen, K.; Mi, M.T. Equol promotes rat osteoblast proliferation and differentiation through activating estrogen receptor. Genet. Mol. Res. 2014, 13, 5055–5063. [Google Scholar] [CrossRef]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.J.; Ha, Y.C.; Park, Y. High dietary sodium intake is associated with low bone mass in postmenopausal women: Korea National Health and Nutrition Examination Survey, 2008–2011. Osteoporos. Int. 2017, 28, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Nowson, C.A. A moderate-sodium DASH-type diet improves mood in postmenopausal women. Nutrition 2012, 28, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Lee, K.B.; Bullerman, L.B. Aflatoxin Production by Aspergillus parasiticus and Its Stability During the Manufacture of Korean Soy Paste (Doenjang) and Soy Sauce (Kanjang) by Traditional Method. J. Food Prot. 1988, 51, 938–944. [Google Scholar] [CrossRef]
- Kim, E.K.; Shon, D.H.; Yoo, J.Y.; Ryu, D.; Lee, C.; Kim, Y.B. Natural occurrence of aflatoxins in Korean meju. Food Addit. Contam. 2001, 18, 151–156. [Google Scholar] [CrossRef]
- Song, J.L.; Choi, J.H.; Seo, J.H.; Lim, Y.I.; Park, K.Y. Anti-Colitic effects of kanjangs (fermented soy sauce and sesame sauce) in dextran sulfate sodium-induced colitis in mice. J. Med. Food 2014, 17, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Song, J.L.; Choi, J.H.; Seo, J.H.; Park, K.Y. Fermented Ganjangs (Soy Sauce and Sesame Sauce) Attenuates Colonic Carcinogenesis in Azoxymethane/Dextran Sodium Sulfate-Treated C57BL/6J Mice. J. Med. Food 2018, 21, 905–914. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, M.; Jeong, S.; Yang, H.; Jeong, D. Characteristic analysis and production of short-ripened Korean traditional soy sauce added with rice bran. J. Korean Soc. Food Sci. Nutr. 2014, 43, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Park, S.Y.; Lee, S.J.; Boo, Y.C.; Choi, J.Y.; Kim, J.E. Ucma, a direct transcriptional target of Runx2 and Osterix, promotes osteoblast differentiation and nodule formation. Osteoarthr. Cartil. 2015, 23, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Litwak, S.A.; Wilson, J.L.; Chen, W.; Garcia-Rudaz, C.; Khaksari, M.; Cowley, M.A.; Enriori, P.J. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology 2014, 155, 4447–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S.; Moon, B.R. Fermented soybeans, Chungkookjang, prevent hippocampal cell death and beta-cell apoptosis by decreasing pro-inflammatory cytokines in gerbils with transient artery occlusion. Exp. Biol. Med. 2015, 241, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Yuan, H.; Zhang, T.; Wu, X.; Huang, S.K.; Cho, S.M. Long-term silk peptide intake promotes skeletal muscle mass, reduces inflammation, and modulates gut microbiota in middle-aged female rats. Biomed Pharm. 2021, 137, 111415. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, E.S.; Kim, D.B.; Kang, S. Low dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E99–E109. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kang, S.; Hua, C.S.; Ting, Z.; Park, S. Synbiotic effects of β-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 2017, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Zhang, T.; Yue, Y.; Wu, X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int. J. Mol. Sci. 2022, 23, 5935. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, T.; Wu, X.G.; Kim, M.J.; Kim, Y.H.; Yang, E.S.; Yoon, Y.S.; Park, S. Aqueous Blackcurrant Extract Improves Insulin Sensitivity and Secretion and Modulates the Gut Microbiome in Non-Obese Type 2 Diabetic Rats. Antioxidants 2021, 10, 756. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2021, 60, 1907–1919. [Google Scholar] [CrossRef]
- Rhee, M.-Y.; Kim, J.-H.; Kim, Y.-S.; Chung, J.-W.; Bae, J.-H.; Nah, D.-Y.; Kim, Y.-K.; Lee, M.-M.; Lim, C.-Y.; Byun, J.-E.; et al. High sodium intake in women with metabolic syndrome. Kor. Circ. J. 2014, 44, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Shojaei-Zarghani, S.; Safarpour, A.R.; Fattahi, M.R.; Keshtkar, A. Sodium in relation with nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Food Sci. Nutr. 2022, 10, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Yang, A.R.; Kim, M.Y.; McCurdy, S.; Boisvert, W.A. Natural sea salt consumption confers protection against hypertension and kidney damage in Dahl salt-sensitive rats. Food Nutr. Res. 2017, 61, 1264713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.Y.; Ok, H.E.; Lee, S.Y.; Jeong, A.Y.; Jeong, T.K.; Chun, H.S. Simple chromatographic determination of aflatoxins in Korean fermented soybean products doenjang, ganjang, and gochujang, with comparison of derivatization methods. Food Sci. Biotech. 2022, 31, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Han, D.M.; Kim, H.M.; Park, D.; Jeong, D.M.; Kang, H.A.; Jeon, C.O. Metabolic Features of Ganjang (a Korean Traditional Soy Sauce) Fermentation Revealed by Genome-Centered Metatranscriptomics. Msystems 2021, 6, e00441-21. [Google Scholar] [CrossRef]
- Song, D.H.; Chun, B.H.; Lee, S.; Son, S.Y.; Reddy, C.K.; Mun, H.I.; Jeon, C.O.; Lee, C.H. Comprehensive Metabolite Profiling and Microbial Communities of Doenjang (Fermented Soy Paste) and Ganjang (Fermented Soy Sauce): A Comparative Study. Foods 2021, 10, 641. [Google Scholar] [CrossRef]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Schulman, I.H.; Aranda, P.; Raij, L.; Veronesi, M.; Aranda, F.J.; Martin, R. Surgical Menopause Increases Salt Sensitivity of Blood Pressure. Hypertension 2006, 47, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-M.; Kim, T.-H.; Lee, H.-H.; Lee, S.H.; Wang, T. Postmenopausal hypertension and sodium sensitivity. J. Menopausal Med. 2014, 20, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Song, J.-L.; Park, E.-S.; Do, M.-S.; Park, K.-Y. Korean solar salts reduce obesity and alter its related markers in diet-induced obese mice. Nutr. Res. Prac. 2016, 10, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Yan, Y.; Gu, Y.; Zhang, Y. Strategy for sodium-salt substitution: On the relationship between hypertension and dietary intake of cations. Food Res. Int. 2022, 156, 110822. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Kim, M.J.; Park, S.H.; Song, E.J.; Nam, Y.D.; Ahn, J.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Bioavailability of Isoflavone Metabolites After Korean Fermented Soybean Paste (Doenjang) Ingestion in Estrogen-Deficient Rats. J. Food Sci. 2018, 83, 2212–2221. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Daily, J.W.; Lee, G.H.; Ryu, M.S.; Yang, H.J.; Jeong, S.Y.; Qiu, J.Y.; Zhang, T.; Park, S. Short-Term Fermented Soybeans with Bacillus amyloliquefaciens Potentiated Insulin Secretion Capacity and Improved Gut Microbiome Diversity and Intestinal Integrity To Alleviate Asian Type 2 Diabetic Symptoms. J. Agric. Food Chem. 2020, 68, 13168–13178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ryu, M.S.; Wu, X.; Yang, H.J.; Jeong, S.J.; Seo, J.W.; Jeong, D.Y.; Park, S. Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut-Brain Axis in Artery-Occluded Gerbils. Foods 2021, 10, 2697. [Google Scholar] [CrossRef]










| Group | Water (%) | Biogenic Amines(mg/kg) | Na (wt%) | Total Aflatoxin (μg/kg) | Bacteria (%) | ||
|---|---|---|---|---|---|---|---|
| Histamine | Tyramine | Beneficial | Pathogenic | ||||
| K-HBLB | 66.2 ± 0.55 d | 7.60 ± 0.18 d | 33.3 ± 0.64 d | 8.11 ± 0.18 a | N.D. | 79.0 ± 5.14 a | 0.023 ± 0.04 c |
| K-HBHB | 72.9 ± 0.96 b | 191 ± 3.05 b | 1150 ± 0.31 a | 5.78 ± 0.10 c | N.D. | 72.4 ± 6.12 a | 0.001 ± 0.0002 d |
| K-LBLB | 70.5 ± 0.50 c | 29.6 ± 0.21 c | 155 ± 0.62 c | 7.46 ± 0.08 b | N.D. | 36.6 ± 1.64 b | 0.19 ± 0.02 b |
| K-LBHB | 74.2 ± 0.59 a | 214 ± 0.92 a | 1009 ± 0.84 b | 6.19 ± 0.15 c | N.D. | 1.94 ± 1.78 c | 33.5 ± 2.74 a |
| Control | HBLB | HBHB | LBLB | LBHB | Normal-Control | |
|---|---|---|---|---|---|---|
| Uterine weight (g) | 0.12 ± 0.01 b | 0.12 ± 0.01 b | 0.15 ± 0.02 b | 0.13 ± 0.01 b | 0.16 ± 0.02 b | 0.79 ± 0.07 a |
| Serum 17β-estradiol (pg/mL) | 1.42 ± 0.24 b | 1.46 ± 0.27 b | 1.59 ± 0.21 b | 1.43 ± 0.29 b | 1.55 ± 0.29 b | 6.72 ± 0.98 a |
| Serum aldosterone (ng/dL) | 14.8 ± 2.3 d | 22.5 ± 2.8 b | 22.9 ± 2.7 b | 19.1 ± 2.6 c | 21.4 ± 3.2 bc | 28.7 ± 3.5 a |
| Final weight (g) | 397 ± 14.0 a | 368 ± 8.82 b | 349 ± 8.14 c | 372 ± 9.97 b | 373 ± 11.0 b | 316 ± 6.76 d |
| Body weight gain (g) | 240 ± 10.7 a | 217 ± 6.99 b | 211 ± 7.69 c | 240 ± 8.74 a | 224 ± 9.12 b | 175 ± 6.03 d |
| Uterine fat (g) | 20.1 ± 2.38 a | 14.4 ± 2.38 c | 14.5 ± 1.10 c | 19.0 ± 1.98 ab | 19.0 ± 1.71a b | 14.0 ± 1.37 c |
| Retroperitoneal fat (g) | 8.22 ± 0.63 a | 7.27 ± 0.84 b | 6.26 ± 0.65 c | 8.55 ± 0.88 a | 7.51 ± 0.81 b | 5.47 ± 0.48 c |
| Visceral fat (% of bw) | 7.3 ± 0.56 a | 5.9 ± 0.53 b | 5.9 ± 0.38 b | 7.2 ± 0.50 a | 7.2 ± 0.55 a | 6.1 ± 0.39 b |
| Food intake (g/day) | 14.1 ± 0.93 | 14.6 ± 1.51 | 14.4 ± 1.33 | 15.2 ± 1.19 | 14.3 ± 0.98 | 14.3 ± 1.05 |
| Food efficiency (%) | 17.1 ± 0.63 a | 15.1 ± 0.84 b | 14.8 ± 0.59 b | 15.8 ± 0.58 b | 16.6 ± 0.71 ab | 13.2 ± 0.46 c |
| Control | HBLB | HBHB | LBLB | LBHB | Normal-Control | |
|---|---|---|---|---|---|---|
| Serum glucose (mg/dL) | 141 ± 6.51 a | 130 ± 7.20 b | 132 ± 3.17 b | 143 ± 5.61 a | 137 ± 7.02 ab | 113 ± 3.57 c |
| Serum insulin (mg/dL) | 2.04 ± 0.31 a | 1.10 ± 0.30 b | 1.25 ± 0.31 b | 2.10 ± 0.37 a | 1.51 ± 0.38 ab | 0.99 ± 0.16 b |
| HOMA-IR | 12.8 ± 1.12 a | 6.31 ± 0.67 c | 7.11 ± 0.55 c | 13.4 ± 0.10 a | 9.27 ± 0.90 b | 4.97 ± 0.30 d |
| Serum total chol (mg/dL) | 123 ± 7.29 a | 116 ± 4.69 a | 115 ± 5.62 b | 102 ± 9.28 c | 103 ± 4.62 c | 118 ± 5.67 a |
| Serum HDL (mg/dL) | 28.8 ± 2.08 d | 56.3 ± 5.46 b | 48.3 ± 2.47 c | 34.8 ± 3.64 c | 37.3 ± 2.21 c | 68.4 ± 5.07 a |
| Serum TG (mg/dL) | 73.7 ± 4.36 a | 70.2 ± 4.12 a | 62.9 ± 2.8 b | 70.6 ± 6.04 ab | 64.8 ± 2.6 b | 54.3 ± 3.04 c |
| Serum LDL (mg/dL) | 79.6 ± 6.27 a | 43.0 ± 3.66 c | 54.3 ± 5.26 b | 53.0 ± 3.39 b | 51.6 ± 3.92 b | 38.6 ± 3.97 d |
| Serum TNF-α (pg/mL) | 56.8 ± 3.9 a | 46.1 ± 3.7 c | 50.3 ± 3.6 b | 52.5 ± 4.2 ab | 51.4 ± 4.1 b | 44.4 ± 3.8 c |
| Serum malondiladhyde (μM) | 43.8 ± 3.8 a | 38.8 ± 3.4 b | 39.5 ± 3.5 b | 40.4 ± 3.3 ab | 41.3 ± 3.8 ab | 34.5 ± 3.6 c |
| Control | HBLB | HBHB | LBLB | LBHB | Normal-Control | |
|---|---|---|---|---|---|---|
| Serum AST (IU/L) | 51.9 ± 5.32 a | 34.1 ± 4.17 b | 28.3 ± 7.22 b | 21.0 ± 5.77 c | 29.7 ± 8.88 b | 20.0 ± 1.66 c |
| Serum ALT (IU/L) | 36.6 ± 6.54 a | 26.3 ± 2.99 b | 15.4 ± 1.82 d | 22.8 ± 2.00 c | 20.1 ± 2.41 c | 18.3 ± 2.66 d |
| Liver glycogen (mg/g tissue) | 68.0 ± 6.90 c | 75.2 ± 7.20 b | 85.6 ± 9.0 a | 68.5 ± 3.71 c | 81.9 ± 4.42 a | 84.5 ± 3.93 a |
| Liver triglyceride (mg/g tissue) | 45.6 ± 6.24 a | 16.7 ± 3.18 d | 18.8 ± 2.66 d | 23.2 ± 3.50 c | 28.6 ± 8.12 b | 13.0 ± 3.40 d |
| Liver relative mRNA of PPAR-γ (AU) | 1 c | 2.45 ± 0.45 a | 1.99 ± 0.43 b | 1.21 ± 0.34 c | 0.89 ± 0.15 c | 2.53 ± 0.49 a |
| Liver relative mRNA of FAS (AU) | 1 a | 0.43 ± 0.09 c | 0.60 ± 0.16 b | 0.66 ± 0.14 b | 0.93 ± 0.17 a | 0.37 ± 0.08 c |
| Liver relative mRNA of SREBP-1c (AU) | 1 | 0.94 ± 0.18 | 0.99 ± 0.17 | 0.89 ± 0.14 | 0.91 ± 0.15 | 0.94 ± 0.17 |
| Control | HBLB | HBHB | LBLB | LBHB | Normal-Control | |
|---|---|---|---|---|---|---|
| Fatty acid degradation | 0.252 ± 0.003 c | 0.270 ± 0.003 b | 0.285 ± 0.004 a | 0.273 ± 0.004 a | 0.280 ± 0.003 a | 0.260 ± 0.005 c |
| Primary bile acid biosynthesis | 0.0306 ± 0.0019 b | 0.0439 ± 0.002 a | 0.0346 ± 0.002 a | 0.0439 ± 0.004 a | 0.0461 ± 0.004 a | 0.0275 ± 0.001 b |
| Biosynthesis of unsaturated fatty acids | 0.003 ± 0.004 b | 0.003 ± 0.008 b | 0.010 ± 0.001 a | 0.007 ± 0.001 ab | 0.003 ± 0.001 b | 0.007 ± 0.001 ab |
| cAMP signaling pathway | 0.008 ± 0.0002 bc | 0.0007 ± 0.0001 c | 0.003 ± 0.0003 a | 0.001 ± 0.0002 b | 0.001 ± 0.0002 b | 0.002 ± 0.0004 ab |
| Glutathione metabolism | 0.2525 ± 0.005 b | 0.3031 ± 0.006 a | 0.291 ± 0.006 a | 0.293 ± 0.008 a | 0.3118 ± 0.005 a | 0.2574 ± 0.005 b |
| Polycyclic aromatic hydrocarbon degradation | 0.0015 ± 0.0003 c | 0.0014 ± 0.0003 c | 0.0059 ± 0.0005 a | 0.0028 ± 0.0004 b | 0.0021 ± 0.0005 b | 0.0038 ± 0.0001 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Zhang, T.; Yue, Y.; Jeong, S.-J.; Ryu, M.-S.; Wu, X.; Yang, H.-J.; Jeong, D.-Y. Alleviation of Metabolic Disturbance by Substituting Kanjang High in Bacillus for Salt through Modulation of Gut Microbiota in Estrogen-Deficient Rats. Foods 2022, 11, 1951. https://doi.org/10.3390/foods11131951
Park S, Zhang T, Yue Y, Jeong S-J, Ryu M-S, Wu X, Yang H-J, Jeong D-Y. Alleviation of Metabolic Disturbance by Substituting Kanjang High in Bacillus for Salt through Modulation of Gut Microbiota in Estrogen-Deficient Rats. Foods. 2022; 11(13):1951. https://doi.org/10.3390/foods11131951
Chicago/Turabian StylePark, Sunmin, Ting Zhang, Yu Yue, Su-Ji Jeong, Myeong-Seon Ryu, Xuangao Wu, Hee-Jong Yang, and Do-Yeon Jeong. 2022. "Alleviation of Metabolic Disturbance by Substituting Kanjang High in Bacillus for Salt through Modulation of Gut Microbiota in Estrogen-Deficient Rats" Foods 11, no. 13: 1951. https://doi.org/10.3390/foods11131951
APA StylePark, S., Zhang, T., Yue, Y., Jeong, S. -J., Ryu, M. -S., Wu, X., Yang, H. -J., & Jeong, D. -Y. (2022). Alleviation of Metabolic Disturbance by Substituting Kanjang High in Bacillus for Salt through Modulation of Gut Microbiota in Estrogen-Deficient Rats. Foods, 11(13), 1951. https://doi.org/10.3390/foods11131951