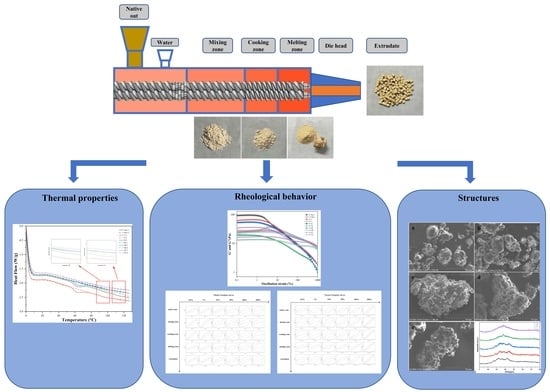
Twin-Screw Extrusion of Oat: Evolutions of Rheological Behavior, Thermal Properties and Structures of Extruded Oat in Different Extrusion Zones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extrusion and Sample Collection
2.3. X-ray Diffraction (XRD)
2.4. Thermal Properties
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Rheological Behavior
2.6.1. Large Amplitude Oscillation Shear Measurement (LAOS)
2.6.2. Small Amplitude Oscillation Shear (SAOS) Measurement
2.6.3. Flow Behavior Measurement
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results
3.1. X-ray Diffraction (XRD)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. DSC
3.4. Rheological Behavior
3.4.1. Flow Behavior
3.4.2. SAOS Behavior
3.4.3. LAOS Behavior
3.4.4. Lissajous Curves Analysis
3.5. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Wavenumber (cm−1) | Peak Assignment |
|---|---|
| 3330 | O-H stretching vibrations, intra- and inter-molecular hydrogen bonding, absorbed water |
| 2927 | C-H stretching vibrations |
| 2853 | Tertiary CH groups |
| 1742 | C=O stretching vibrations |
| 1657 | Amide I absorption (predominantly the C=O stretching) |
| 1533 | Amide II (an N-H bending vibration coupled to C-N stretching) |
| 1459 | C-H deformation in lignin |
| 1412 | C-N stretching vibrations |
| 1373 | Bending vibrations of OH in cellulose and hemicellulose/Symmetric CH3 bending modes of the methyl groups of proteins |
| 1242 | C-O stretching vibrations |
| 1155 | Stretching vibration of C=C and C-O |
| 1080 | C-O deformation |
| 1026 | C-O stretching vibrations |
| 860 | C-H out- of–plane deformation in lignin |
| 707 | CH2 rocking in cellulose |
References
- Singh, R.; De, S.; Belkheir, A. Avena sativa (Oat), A potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Ye, F.Y.; Lei, L.; Zhou, S.Y.; Zhao, G.H. Fabricating low glycaemic index foods: Enlightened by the impacts of soluble dietary fibre on starch digestibility. Trends Food Sci. Technol. 2022, 122, 110–122. [Google Scholar] [CrossRef]
- Rodrigues, E.A.; Badiola, I.; Francesch, M.; Torrallardona, D. Effect of cereal extrusion on performance, nutrient digestibility, and cecal fermentation in weanling pigs1. J. Anim. Sci. 2016, 94, 298–302. [Google Scholar] [CrossRef]
- Wiedmann, W. Extrusion cooking of starches for semi-products. Starch-Stärke 1987, 39, 352–357. [Google Scholar] [CrossRef]
- Schmid, V.; Mayer-Miebach, E.; Behsnilian, D.; Briviba, K.; Karbstein, H.P.; Emin, M.A. Enrichment of starch-based extruded cereals with chokeberry (Aronia melanocarpa) pomace: Influence of processing conditions on techno-functional and sensory related properties, dietary fibre and polyphenol content as well as in vitro digestibility. LWT-Food Sci. Technol. 2022, 154, 112610. [Google Scholar] [CrossRef]
- Ai, Y.; Cichy, K.A.; Harte, J.B.; Kelly, J.D.; Ng, P.K.W. Effects of extrusion cooking on the chemical composition and functional properties of dry common bean powders. Food Chem. 2016, 211, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Zapana, F.; de Bruijn, J.; Vidal, L.; Melin, P.; Gonzalez, M.E.; Cabrera, G.; Williams, P.; Borquez, R. Physical, chemical and nutritional characteristics of puffed quinoa. Int. J. Food Sci. Technol. 2020, 55, 313–322. [Google Scholar] [CrossRef]
- De Pilli, T.; Giuliani, R.; Buleon, A.; Pontoire, B.; Legrand, J. Effects of protein-lipid and starch-lipid complexes on textural characteristics of extrudates based on wheat flour with the addition of oleic acid. Int. J. Food Sci. Technol. 2016, 51, 1063–1074. [Google Scholar] [CrossRef]
- Jiang, R.S.; Xiao, Z.G.; Huo, J.J.; Wang, H.G.; Li, H.; Su, S.; Duan, Y.M.; Gao, Y.Z. Effects of rice bran content on plant-based simulated meat: From the aspects of apparent properties and structural characteristics. Food Chem. 2022, 380, 131842. [Google Scholar] [CrossRef]
- Lai, L.S.; Kokini, J.L. Physicochemical changes and rheological properties of starch during extrusion. Biotechnol. Prog. 1991, 7, 251–266. [Google Scholar] [CrossRef]
- Sinaki, N.Y.; Masatcioglu, M.T.; Paliwal, J.; Koksel, F. Development of cellular high-protein foods: Third-generation yellow pea and red lentil puffed snacks. Foods 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.; Marin, S.; Sanchis, V.; Ramos, A.J. Fate of mycotoxins in cereals during extrusion cooking: A review. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2005, 22, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Liu, L.; Liu, H.Z.; Yoon, A.; Rizvi, S.S.H.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; van den Berg, F.W.J.; Zhang, W.; Czaja, T.P.; Zhang, L.T.; Jespersen, B.M.; Lametsch, R. Differences in physicochemical properties of high-moisture extrudates prepared from soy and pea protein isolates. Food Hydrocoll. 2022, 128, 107540. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT-Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Pansawat, N.; Jangchud, K.; Jangchud, A.; Wuttijumnong, P.; Saalia, F.K.; Eitenmiller, R.R.; Phillips, R.D. Effects of extrusion conditions on secondary extrusion variables and physical properties of fish, rice-based snacks. LWT Food Sci. Technol. 2008, 41, 632–641. [Google Scholar] [CrossRef]
- Vainionpää, J. Modelling of extrusion cooking of cereals using response surface methodology. J. Food Eng. 1991, 13, 1–26. [Google Scholar] [CrossRef]
- Dalbhagat, C.G.; Mahato, D.K.; Mishra, H.N. Effect of extrusion processing on physicochemical, functional and nutritional characteristics of rice and rice-based products: A review. Trends Food Sci. Technol. 2019, 85, 226–240. [Google Scholar] [CrossRef]
- Cai, W.; Diosady, L.L. Model for gelatinization of wheat starch in a twin-screw extruder. J. Food Sci. 1993, 58, 872–875. [Google Scholar] [CrossRef]
- Chen, Q.L.; Zhang, J.C.; Zhang, Y.J.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Zambrano, Y.; Contardo, I.; Moreno, M.C.; Bouchon, P. Effect of extrusion temperature and feed moisture content on the microstructural properties of rice-flour pellets and their impact on the expanded product. Foods 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Lampi, A.-M.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Sarker, M.Z.I.; Elgadir, M.A.; Ferdosh, S.; Akanda, M.J.H.; Aditiawati, P.; Noda, T. Rheological behavior of starch-based biopolymer mixtures in selected processed foods. Starch-Stärke 2013, 65, 73–81. [Google Scholar] [CrossRef]
- Kristiawan, M.; Chaunier, L.; Sandoval, A.J.; Della Valle, G. Extrusion-Cooking and Expansion; Woodhead Publishing and AACC International Press: Cambridge, UK, 2020; pp. 141–167. [Google Scholar]
- Goudoulas, T.B.; Germann, N. Nonlinear rheological behavior of gelatin gels: In situ gels and individual layers. J. Colloid Interface Sci. 2019, 553, 746–757. [Google Scholar] [CrossRef]
- Yazar, G.; Duvarci, O.C.; Tavman, S.; Kokini, J.L. LAOS behavior of the two main gluten fractions: Gliadin and glutenin. J. Cereal Sci. 2017, 77, 201–210. [Google Scholar] [CrossRef]
- Carriere, C.J.; Thomas, A.J.; Inglett, G.E. Prediction of the nonlinear transients and oscillatory rheological behavior of flour suspensions using a strain-separable integral constitutive equation. Carbohydr. Polym. 2002, 47, 219–231. [Google Scholar] [CrossRef]
- Yin, X.; Ma, Z.; Hu, X.; Li, X.; Boye, J.I. Molecular rearrangement of Laird lentil (Lens culinaris Medikus) starch during different processing treatments of the seeds. Food Hydrocoll. 2018, 79, 399–408. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Zhou, L.; Ji, N.; Xiong, L.; Sun, Q. Green preparation of debranched starch nanoparticles with different crystalline structures by electrostatic spraying. Food Hydrocoll. 2022, 127, 107513. [Google Scholar] [CrossRef]
- Moisio, T.; Forssell, P.; Partanen, R.; Damerau, A.; Hill, S.E. Reorganisation of starch, proteins and lipids in extrusion of oats. J. Cereal Sci. 2015, 64, 48–55. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.Y.; Xie, J.; Su, E.Y.; Wan, Z.L.; Sagis, L.M.C.; Yang, X.Q. Large amplitude oscillatory shear (LAOS) for nonlinear rheological behavior of heterogeneous emulsion gels made from natural supramolecular gelators. Food Res. Int. 2021, 140, 110076. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, C.; Wang, N.; Chen, Y.; Yu, J.J.; Zheng, X.C.; Li, S.H.; Chen, Y. Developing a new modification technology of oat flour based on differential pressure explosion puffing. LWT-Food Sci. Technol. 2021, 141, 110967. [Google Scholar] [CrossRef]
- González-Gutiérrez, J.; Partal, P.; García-Morales, M.; Gallegos, C. Effect of processing on the viscoelastic, tensile and optical properties of albumen/starch-based bioplastics. Carbohydr. Polym. 2011, 84, 308–315. [Google Scholar] [CrossRef]
- Dries, D.M.; Gomand, S.V.; Delcour, J.A.; Goderis, B. V-type crystal formation in starch by aqueous ethanol treatment: The effect of amylose degree of polymerization. Food Hydrocoll. 2016, 61, 649–661. [Google Scholar] [CrossRef]
- Dong, R.; Niu, Q.; Zhang, K.; Hu, X.; Bu, Y. The effect of retrogradation time and ambient relative humidity on the quality of extruded oat noodles. Food Sci. Nutr. 2020, 8, 2940–2949. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Smith, C.; Zhou, Y.; Ratnayake, R.M.W.S. Physicochemical properties of Canadian oat starches. Carbohydr. Polym. 2003, 52, 253–261. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Kadivar, M. Properties of whey protein isolate nanocomposite films reinforced with nanocellulose isolated from oat husk. Int. J. Biol. Macromol. 2016, 91, 1134–1140. [Google Scholar] [CrossRef]
- Yang, S.N.; Zhang, Q.L.; Yang, H.Y.; Shi, H.M.; Dong, A.C.; Wang, L.; Yu, S.N. Progress in infrared spectroscopy as an efficient tool for predicting protein secondary structure. Int. J. Biol. Macromol. 2022, 206, 175–187. [Google Scholar] [CrossRef]
- Morales-Sanchez, E.; Cabrera-Ramirez, A.H.; Gaytan-Martinez, M.; Mendoza-Zuvillaga, A.L.; Velazquez, G.; Mendez-Montealvo, M.G.; Rodriguez-Garcia, M.E. Heating-cooling extrusion cycles as a method to improve the physicochemical properties of extruded corn starch. Int. J. Biol. Macromol. 2021, 188, 620–627. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wojtowicz, A.; Sujak, A. Effect of composition and processing conditions on selected properties of potato-based pellets and microwave-expanded snacks supplemented with fresh beetroot pulp. Pol. J. Food Nutr. Sci. 2021, 71, 211–224. [Google Scholar] [CrossRef]
- Paton, D. Differential scanning calorimetry of oat starch pastes. Cereal Chem. 1987, 64, 394–399. [Google Scholar]
- Yang, Z.; Zhou, Y.; Xing, J.-J.; Guo, X.-N.; Zhu, K.-X. Influence of extrusion on storage quality of dried oat noodles: Lipid degradation and off-flavours. J. Cereal Sci. 2021, 101, 103316. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.J.; Zhang, B.; Drago, S.R.; Zhang, J.C. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, E.; Yao, Y. New techniques in structural tailoring of starch functionality. Annu. Rev. Food Sci. Technol. 2022, 13, 117–143. [Google Scholar] [CrossRef]
- Sun, G.H.; Liang, T.Q.; Tan, W.Y.; Wang, L.J. Rheological behaviors and physical properties of plasticized hydrogel films developed from kappa-carrageenan incorporating hydroxypropyl methylcellulose. Food Hydrocoll. 2018, 85, 61–68. [Google Scholar] [CrossRef]
- Kristiawan, M.; Della Valle, G.; Reguerre, A.L.; Micard, V.; Salles, C. Artificial oral processing of extruded pea flour snacks. Food Eng. Rev. 2021, 13, 247–261. [Google Scholar] [CrossRef]
- Cabrera-Ramirez, A.H.; Morales-Sanchez, E.; Mendez-Montealvo, G.; Velazquez, G.; Rodriguez-Garcia, M.E.; Villamiel, M.; Gaytan-Martinez, M. Structural changes in popped sorghum starch and their impact on the rheological behavior. Int. J. Biol. Macromol. 2021, 186, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, L.; Hounsou, M.; Matignon, B.; Ricci, J.; Madode, Y.; Hounhouigan, J.; Akissoe, N.; Mestres, C. Role of dough viscoelastic properties and rice variety in the thermal expansion and the quality of unconventional rice-based bread: Case of steamed-cooked ‘Ablo’. Int. J. Food Sci. Technol. 2021, 56, 4615–4626. [Google Scholar] [CrossRef]
- Jian, T.; Ding, X.L. Relationship between functional-properties and macromolecular modifications of extruded corn starch. Cereal Chem. 1994, 71, 364–369. [Google Scholar]
- Almeida, R.L.J.; Pereira, T.D.; Almeida, R.D.; Santiago, A.M.; Marsiglia, W.; Nabeshima, E.H.; Conrado, L.D.; de Gusmao, R.P. Rheological and technological characterization of red rice modified starch and jaboticaba peel powder mixtures. Sci. Rep. 2021, 11, 9284. [Google Scholar] [CrossRef]
- Huamani-Melendez, V.J.; Mauro, M.A.; Darros-Barbosa, R. Physicochemical and rheological properties of aqueous Tara gum solutions. Food Hydrocoll. 2021, 111, 106195. [Google Scholar] [CrossRef]
- Dong, H.M.; Zhang, Q.; Gao, J.; Chen, L.Y.; Vasanthan, T. Comparison of morphology and rheology of starch nanoparticles prepared from pulse and cereal starches by rapid antisolvent nanoprecipitation. Food Hydrocoll. 2021, 119, 106828. [Google Scholar] [CrossRef]
- Xie, Y.S.; Yu, X.L.; Wang, Z.M.; Yu, C.X.; Prakash, S.; Dong, X.P. The synergistic effects of myofibrillar protein enrichment and homogenization on the quality of cod protein gel. Food Hydrocoll. 2022, 127, 107468. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of fish gelatin and gum arabic interactions on concentrated emulsion large amplitude oscillatory shear behavior and tribological properties. Food Hydrocoll. 2018, 79, 518–525. [Google Scholar] [CrossRef]
- Vasquez, C.; Henriquez, G.; Lopez, J.V.; Penott-Chang, E.K.; Sandoval, A.J.; Muller, A.J. The effect of composition on the rheological behavior of commercial chocolates. LWT-Food Sci. Technol. 2019, 111, 744–750. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.H.; Sun, K.F.; Chang, C.; Liu, W.F. Effects of covalent interactions and gel characteristics on soy protein-tannic acid conjugates prepared under alkaline conditions. Food Hydrocoll. 2021, 112, 106293. [Google Scholar] [CrossRef]
- Lazou, A.; Krokida, M. Functional properties of corn and corn–lentil extrudates. Food Res. Int. 2010, 43, 609–616. [Google Scholar] [CrossRef]
- Anvari, M.; Tabarsa, M.; Joyner, H.S. Large amplitude oscillatory shear behavior and tribological properties of gum extracted from Alyssum homolocarpum seed. Food Hydrocoll. 2018, 77, 669–676. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Sim, H.G.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear behavior of complex fluids investigated by a network model: A guideline for classification. J. Non-Newton. Fluid Mech. 2003, 112, 237–250. [Google Scholar] [CrossRef]
- PhanThien, N.; SafariArdi, M.; MoralesPatino, A. Oscillatory and simple shear flows of a flour-water dough: A constitutive model. Rheol. Acta 1997, 36, 38–48. [Google Scholar] [CrossRef]
- Niu, F.G.; Li, M.Y.; Fan, J.M.; Kou, M.X.; Han, B.J.; Pan, W.C. Structural characteristics and digestibility of bovine skin protein and corn starch extruded blend complexes. J. Food Sci. Technol.-Mysore 2020, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zheng, B.; Tang, Y.K.; Chen, L. Starch concentration is an important factor for controlling its digestibility during hot-extrusion 3D printing. Food Chem. 2022, 379, 132180. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Hyun, K.; Ahn, K.H.; Lee, S.J. A geometrical interpretation of large amplitude oscillatory shear response. J. Rheol. 2005, 49, 747–758. [Google Scholar] [CrossRef]
- Joyner, H.S. Nonlinear (large-amplitude oscillatory shear) rheological properties and their impact on food processing and quality. Annu. Rev. Food Sci. Technol. 2021, 12, 591–609. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Sagis, L.M.C.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Small and large oscillatory shear properties of concentrated proteins. Food Hydrocoll. 2021, 110, 106172. [Google Scholar] [CrossRef]
- Ptaszek, P.; Kabzinski, M.; Ptaszek, A.; Kaczmarczyk, K.; Kruk, J.; Bienczak, A. The analysis of the influence of xanthan gum and apple pectins on egg white protein foams using the large amplitude oscillatory shear method. Food Hydrocoll. 2016, 54, 293–301. [Google Scholar] [CrossRef]
- Zhou, X.; Xing, Y.R.; Meng, T.T.; Li, J.X.; Chang, Q.; Zhao, J.W.; Jin, Z.Y. Preparation of V-type cold water-swelling starch by ethanolic extrusion. Carbohydr. Polym. 2021, 271, 118400. [Google Scholar] [CrossRef]
- Chanvrier, H.; Colonna, P.; Della Valle, G.; Lourdin, D. Structure and mechanical behaviour of corn flour and starch–zein based materials in the glassy state. Carbohydr. Polym. 2005, 59, 109–119. [Google Scholar] [CrossRef]








| Ingredients (g/100g) | Water | Protein | Fat | Carbohydrate | Sodium |
| 8.5 | 12.6 | 3.1 | 70.6 | 0.011 |
| Water Content | Extrusion Zones | Position of the Diffraction Peaks | RC | |||
|---|---|---|---|---|---|---|
| 15% | I | 13.82 ± 0.57 a | 16.81 ± 0.66 a | 18.11 ± 0.35 bcde | 21.50 ± 0.68 ab | 0.29 ± 0.06 a |
| II | 13.50 ± 0.19 a | 16.63 ± 0.51 a | 17.93 ± 0.14 de | 21.11 ± 0.19 b | 0.33 ± 0.01 a | |
| III | - | - | 17.93 ± 0.05 de | 21.00 ± 0.71 b | 0.23 ± 0.01b | |
| Ⅳ | - | - | 18.15 ± 0.12 bcde | - | 0.19 ± 0.02 b | |
| 18% | I | 13.84 ± 0.90 a | 16.78 ± 0.74 ab | 19.01 ± 0.37 ab | 22.2 ± 0.20 a | 0.30 ± 0.02 a |
| II | 13.97 ± 0.81 a | 16.85 ± 0.25 ab | 19.25 ± 0.19 a | 21.72 ± 0.73 ab | 0.30 ± 0.01 a | |
| III | - | - | 18.74 ± 0.79 abcd | 21.80 ± 0.28 ab | 0.29 ± 0.02 a | |
| Ⅳ | - | - | 19.16 ± 0.12 a | - | 0.18 ± 0.01 b | |
| 20% | I | 14.12 ± 0.22 a | 17.04 ± 0.15 a | 18.95 ± 0.35 abc | 21.97 ± 0.47 ab | 0.29 ± 0.03 a |
| II | 13.75 ± 0.27 a | 17.15 ± 0.38 a | 19.23 ± 0.14 a | 21.93 ± 0.11 ab | 0.31 ± 0.01 a | |
| III | - | - | 17.81 ± 0.19 e | - | 0.27 ± 0.02 ab | |
| Ⅳ | - | - | 18.03 ± 0.27 cde | - | 0.22 ± 0.02 b | |
| NM | 13.29 ± 0.13 a | 16.12 ± 0.25 b | 18.78 ± 0.76 abcd | 21.81 ± 0.69 ab | 0.28 ± 0.01 ab | |
| Extrusion Zones | Secondary Structure (%) | |||
|---|---|---|---|---|
| α-Helixes | β-Sheets | β-Turns | Random Coils | |
| I | 34.49 ± 0.01 c | 24.83 ± 0.01 c | 26.49 ± 0.02 b | 14.19 ± 0.10 b |
| II | 36.10 ± 0.01 b | 20.37 ± 0.03 d | 28.97 ± 0.07 a | 14.56 ± 0.04 b |
| III | 38.18 ± 0.28 a | 27.75 ± 0.15 b | 15.48 ± 0.02 e | 18.54 ± 0.06 a |
| Ⅳ | 31.20 ± 0.59 e | 30.02 ± 0.86 a | 24.51 ± 0.03 d | 14.27 ± 0.29 b |
| NM | 32.79 ± 0.03 d | 27.20 ± 0.01 b | 25.76 ± 0.07 c | 14.25 ± 0.10 b |
| Samples | Peak 1 | Peak 2 | Peak 3 | Secondary Scanning | |||||
|---|---|---|---|---|---|---|---|---|---|
| Peak Temperature (°C) | Enthalpy (J/g) | Peak Temperature (°C) | Enthalpy (J/g) | Peak Temperature (°C) | Enthalpy (J/g) | Peak Temperature (°C) | Enthalpy (J/g) | ||
| 15% | I | 62.24 ± 0.28 g | 9.22 ± 0.18 a | 97.32 ± 0.12 a | 1.84 ± 0.56 a,b,c | 115.23 ± 0.05 a | 0.51 ± 0.06 b,c,d | 100.92 ± 0.07 a,b,c | 4.15 ± 0.17 d |
| II | 63.27 ± 0.31 e,f,g | 7.61 ± 0.23 b,c | 96.83 ± 0.26 a,b | 1.6 ± 0.25 a,b,c | 115.63 ± 0.14 a | 0.88 ± 0.19 a | 101.38 ± 0.38 a | 5.54 ± 0.86 b,c | |
| III | 68.61 ± 0.11 a,b | 1.06 ± 0.17 h,i | 96.56 ± 0.17 a,b,c | 0.16 ± 0.03 e | 116.31 ± 0.33 a | 0.18 ± 0.05 f | 98.82 ± 0.05 c,d,e | 6.71 ± 0.41 a | |
| Ⅳ | 66.37 ± 3.06 b,c,d | 1.57 ± 0.38 g,h | 96.12 ± 0.49 a,b,c | 0.19 ± 0.1 e | 114.84 ± 1.75 a | 0.27 ± 0.08 d,e,f | 98.85 ± 0.24 b,c,d,e | 6.21 ± 0.22 a,b | |
| 18% | I | 62.49 ± 0.14 f,g | 7.03 ± 0.05 d | 96.01 ± 0.59 a,b,c | 1.38 ± 0.31 b,c,d | 116.49 ± 1.64 a | 0.63 ± 0.17 a,b | 97.99 ± 0.07 d,e,f | 4.55 ± 0.25 c,d |
| II | 64.83 ± 0.95 d,e,f | 5.99 ± 0.06 e | 97.04 ± 0.26 a | 1.03 ± 0.94 c,d | 115.31 ± 2.90 a | 0.36 ± 0.19 b,c,d,e,f | 99.23 ± 2.55 a,b,c,d | 1.78 ± 0.08 e,f | |
| III | 67.97 ± 0.10 a,b,c | 2.28 ± 0.04 f | 95.70 ± 0.35 b,c | 0.55 ± 0.3 d,e | 115.03 ± 3.37 a | 0.35 ± 0.13 c,d,e,f | 96.26 ± 0.37 f,g | 1.17 ± 0.39 f | |
| Ⅳ | 65.65 ± 0.47 c,d | 1.04 ± 0.10 i | 96.29 ± 0.95 a,b,c | 1.18 ± 0.11 c,d | 113.2 ± 0.28 a | 0.49 ± 0.01 b,c,d,e | 96.06 ± 0.90 f,g | 1.68 ± 0.09 e,f | |
| 20% | I | 62.60 ± 0.49 f,g | 8.77 ± 0.04 a | 96.78 ± 0.57 a,b | 2.31 ± 0.06 a | 114.55 ± 0.12 a | 0.61 ± 0.07 a,b,c | 97.48 ± 0.57 d,e,f,g | 3.72 ± 0.22 d |
| II | 63.35 ± 0.25 e,f,g | 7.30 ± 0.28 c,d | 96.79 ± 0.50 a,b | 1.52 ± 0.19 a,b,c | 114.93 ± 0.12 a | 0.43 ± 0.16 b,c,d,e,f | 101.03 ± 0.28 a,b | 1.81 ± 0.08 e,f | |
| III | 69.22 ± 1.46 a | 0.72 ± 0.15 i | 96.47 ± 0.33 a,b,c | 1.25 ± 0.09 c,d | 114.15 ± 0.09 a | 0.23 ± 0.09 e,f | 99.40 ± 0.85 a,b,c,d | 2.39 ± 0.67 e | |
| Ⅳ | 66.65 ± 0.63 c,d,e | 1.58 ± 0.44 g | 96.73 ± 0.17 a,b | 2.19 ± 0.35 a,b | 115.65 ± 0.03 a | 0.31 ± 0.04 d,e,f | 96.68 ± 0.24 e,f,g | 2.52 ± 0.64 e | |
| NM | 62.58 ± 0.35 f,g | 7.86 ± 0.03 b | 95.37 ± 0.83 c | 1.15 ± 0.08 c,d | 112.92 ± 1.23 a | 0.84 ± 0.01 a | 95.74 ± 1.29 g | 1.74 ± 0.12 e,f | |
| Power Law | Cross | |||||||
|---|---|---|---|---|---|---|---|---|
| Viscosity (Pa) | Rate Index | R2 | Zero-Rate Viscosity (Pa∙s) | Infinite-Rate Viscosity (Pa∙s) | Consistency (s) | Rate Index | R2 | |
| NM | 13.94 ± 0.35 a | −0.58 ± 0.001 b | 0.972 | 15.08 ± 1.68 a | −0.276 ± 0.063 e | 0.40 ± 0.079 a | 0.68 ± 0.025 e | 0.999 |
| I | 10.52 ± 1.23 c | −0.63 ± 0.030 d | 0.973 | 8.23 ± 1.30 c | 0.140 ± 0.050 c | 0.23 ± 0.070 c | 0.95 ± 0.070 d | 0.999 |
| II | 13.26 ± 0.43 b | −0.73 ± 0.003 e | 0.978 | 11.69 ± 0.15 b | 0.151 ± 0.001 b | 0.37 ± 0.003 b | 0.98 ± 0.007 c | 0.999 |
| III | 8.15 ± 0.04 d | −0.61 ± 0.002 c | 0.954 | 5.45 ± 0.04 d | 0.132 ± 0.003 d | 0.14 ± 0.001 e | 1.04 ± 0.003 a | 0.998 |
| Ⅳ | 5.16 ± 0.17 e | −0.57 ± 0.001 a | 0.966 | 3.98 ± 0.39 e | 0.154 ± 0.012 a | 0.17 ± 0.024 d | 1.03 ± 0.049 b | 0.998 |
| Power Law | ||||||
|---|---|---|---|---|---|---|
| K′ (Pa) | n′ | R2 | K″ (Pa) | n″ | R2 | |
| NM | 36.10 ± 0.89 a | 0.23 ± 0.003 d | 0.999 | 15.90 ± 0.85 a | 0.23 ± 0.001 b | 0.996 |
| I | 31.55 ± 0.49 b | 0.21 ± 0.001 e | 0.997 | 13.92 ± 0.68 b | 0.18 ± 0.012 c | 0.977 |
| II | 21.45 ± 0.87 c | 0.26 ± 0.002 c | 0.998 | 10.91 ± 0.37 c | 0.22 ± 0.006 b | 0.980 |
| III | 9.15 ± 0.61 d | 0.38 ± 0.010 a | 0.999 | 6.98 ± 0.32 d | 0.34 ± 0.007 a | 0.999 |
| Ⅳ | 6.90 ± 0.41 e | 0.29 ± 0.008 b | 0.996 | 4.55 ± 0.30 e | 0.25 ± 0.020 b | 0.983 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Wu, M.; Sun, D.; Wei, W.; Yu, H.; Zhang, T. Twin-Screw Extrusion of Oat: Evolutions of Rheological Behavior, Thermal Properties and Structures of Extruded Oat in Different Extrusion Zones. Foods 2022, 11, 2206. https://doi.org/10.3390/foods11152206
Zhou C, Wu M, Sun D, Wei W, Yu H, Zhang T. Twin-Screw Extrusion of Oat: Evolutions of Rheological Behavior, Thermal Properties and Structures of Extruded Oat in Different Extrusion Zones. Foods. 2022; 11(15):2206. https://doi.org/10.3390/foods11152206
Chicago/Turabian StyleZhou, Chengyi, Min Wu, Dongyu Sun, Wenguang Wei, Haoze Yu, and Tong Zhang. 2022. "Twin-Screw Extrusion of Oat: Evolutions of Rheological Behavior, Thermal Properties and Structures of Extruded Oat in Different Extrusion Zones" Foods 11, no. 15: 2206. https://doi.org/10.3390/foods11152206
APA StyleZhou, C., Wu, M., Sun, D., Wei, W., Yu, H., & Zhang, T. (2022). Twin-Screw Extrusion of Oat: Evolutions of Rheological Behavior, Thermal Properties and Structures of Extruded Oat in Different Extrusion Zones. Foods, 11(15), 2206. https://doi.org/10.3390/foods11152206