A Minimally Invasive Approach for Preventing White Wine Protein Haze by Early Enzymatic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes, Chemicals and Must
2.2. Enzymatic Activity Assay and Kinetic Study
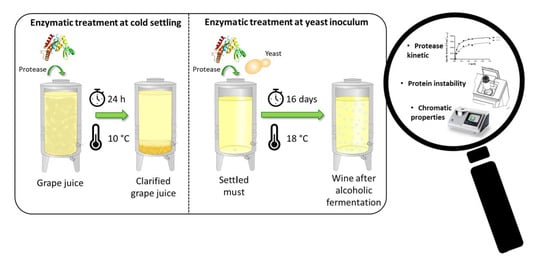
2.3. Enzymatic Treatment and Laboratory-Scale Fermentation Tests
2.4. Heat Stability and Haze-Active (HA) Protein Determination
2.5. Total Protein Content and Electrophoretic Separation (SDS-PAGE)
2.6. Effect of Enzymatic Treatment on Chromatic Characteristics and Phenolics
2.7. Statistical Analysis
3. Results and Discussion
3.1. Enzyme Kinetic Study
3.2. Alcoholic Fermentation Kinetic
3.3. Effect of Enzymatic Treatment on Protein Instability
3.4. Effect of Enzymatic Treatment on Chromatic Characteristics and Phenolics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White wine protein instability: Mechanism, quality control and technological alternatives for wine stabilisation—An overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Harrison, R. Pathogenesis-Related Proteins in Wine and White Wine Protein Stabilization. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Vienna, Austria, 2020. [Google Scholar]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Silva-Barbieri, D.; Salazar, F.N.; López, F.; Brossard, N.; Escalona, N.; Pérez-Correa, J.R. Advances in White Wine Protein Stabilization Technologies. Molecules 2022, 27, 1251. [Google Scholar] [CrossRef]
- Celotti, E.; Barahona, M.S.O.; Bellantuono, E.; Cardona, J.; Roman, T.; Nicolini, G.; Natolino, A. High-power ultrasound on the protein stability of white wines: Preliminary study of amplitude and sonication time. LWT 2021, 147, 111602. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Qi, G.; Smith, P.; Bindon, K.; Vasilev, K. Regeneration of magnetic nanoparticles used in the removal of pathogenesis-related proteins from white wines. Foods 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Mierczynska-Vasilev, A.; Wahono, S.K.; Smith, P.A.; Bindon, K.; Vasilev, K. Using zeolites to protein stabilize white wines. ACS Sustain. Chem. Eng. 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250. [Google Scholar] [CrossRef]
- Millarini, V.; Ignesti, S.; Cappelli, S.; Ferraro, G.; Adessi, A.; Zanoni, B.; Fratini, E.; Domizio, P. Protection of Wine from Protein Haze Using Schizosaccharomyces japonicus Polysaccharides. Foods 2020, 9, 1407. [Google Scholar] [CrossRef]
- Ratnayake, S.; Stockdale, V.; Grafton, S.; Munro, P.; Robinson, A.L.; Pearson, W.; McRae, J.M.; Bacic, A. Carrageenans as heat stabilisers of white wine. Aust. J. Grape Wine Res. 2019, 25, 439–450. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Esti, M.; Benucci, I.; Lombardelli, C.; Liburdi, K.; & Garzillo, A.M.V. Papain from papaya (Carica papaya L.) fruit and latex: Preliminary characterization in alcoholic–acidic buffer for wine application. Food Bioprod. Process. 2013, 91, 595–598. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of wine inhibitors on the proteolytic activity of papain from Carica papaya L. latex. Biotechnol. Prog. 2015, 31, 48–54. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of free and immobilised stem bromelain on protein haze in white wine. Aust. J. Grape Wine Res. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Technol. 2016, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EEC) No 2676/90 of 17 September 1990 Determining Community Methods for the Analysis of Wines; European Commission: Brussels, Belgium, 1990; Volume 272, pp. 1–192. [Google Scholar]
- Lopez, M.; Edens, L. Effective prevention of chill-haze in beer using an acid proline-specific endoprotease from Aspergillus niger. J. Agric. Food Chem. 2005, 53, 7944–7949. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M. Arginase Activity Characterization During Alcoholic Fermentation by Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeast. Food Bioprocess Technol. 2021, 14, 1996–2003. [Google Scholar] [CrossRef]
- Benucci, I.; Fiorelli, V.; Lombardelli, C.; Liburdi, K.; Esti, M. Kinetic characterization of arginase from Saccharomyces cerevisiae during alcoholic fermentation at different temperatures. LWT 2017, 82, 268–273. [Google Scholar] [CrossRef]
- Vincenzi, S.; Marangon, M.; Tolin, S.; Curioni, A. Protein evolution during the early stages of white winemaking and its relations with wine stability. Aust. J. Grape Wine Res. 2011, 17, 20–27. [Google Scholar] [CrossRef]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein− polyphenol haze in beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Cacciotti, I.; Esti, M. Multi-enzymatic systems immobilized on chitosan beads for pomegranate juice treatment in fluidized bed reactor: Effect on haze-active molecules and chromatic properties. Food Bioprocess Technol. 2019, 12, 1559–1572. [Google Scholar] [CrossRef]
- Becchetti, R.; Sanvito, M. Metodi di Analisi dei Vini e delle Bevande Spiritose; Gibertini Elettronica: Milan, Italy, 1999. [Google Scholar]
- de Esteban, M.L.G.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A. Enhancing Excel capability to perform statistical analyses in agriculture applied research. In Computational Statistics and Data Analysis–Statistical Software Newsletters; International Association for Statistical Computing: The Hague, The Netherlands, 2006. [Google Scholar]
- Kang, C.; Yu, X.W.; Xu, Y. Purification and characterization of a prolyl endopeptidase isolated from Aspergillus oryzae. J. Ind. Microbiol. Biotechnol. 2014, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhao, H.; Zhao, M. Proteases supplementation to high gravity worts enhances fermentation performance of brewer’s yeast. Biochem. Eng. J. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Mathias, T.R.S.; Lopes, M.C.R.D.; Oliveira, C.A.; Carvalho, R.C.; Marques, F.F.C.; Sérvulo, E.F.C. Influence of mashing profile curve and addition of proteases on the composition of the wort and beer. MOJ Food Process. Technol. 2017, 5, 124. [Google Scholar]
- Comuzzo, P.; Voce, S.; Fabris, J.; Cavallaro, A.; Zanella, G.; Karpusas, M.; Kallithraka, S. Effect of the combined application of heat treatment and proteases on protein stability and volatile composition of Greek white wines. OENO One 2020, 54, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Tušek, A.J.; Ganić, K.K. Quality characteristics of white wine: The short-and long-term impact of high power ultrasound processing. Ultrason. Sonochem. 2020, 68, 105194. [Google Scholar] [CrossRef]
- Valášek, P.; Mlček, J.; Adámková, A.; Křivánková, M.; Adámek, M.; Sedláčková, E. Comparison of contents of selected esters, higher alcohols and total content of polyphenolic substances in wines of the varieties’ Chardonnay’ and ‘Riesling’ by vintage. Mitt. Klosterneubg. Rebe Wein Obstb. Fruchteverwert. 2019, 69, 115–123. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E. Trattato di Enologia; Edagricole: Bologna, Italy, 1957. [Google Scholar]







| Description | Duration | Temperature | Reference Sample (Ctrl) | Analysis Time | |
|---|---|---|---|---|---|
| Treatment I | Addition of protease before cold settling prior to AF | 24 h | 10 °C | Grape juice settled with only pectinolytic enzyme | After racking |
| Treatment II | Addition of protease at yeast inoculum | Overall AF duration (16 days) | 18 °C | Clear must after cold settling (with pectinolytic enzyme) fermented without any enzyme addition | Middle AF End AF |
| T = 10 °C | T = 18 °C | |
|---|---|---|
| Vmax (I.U.mg−1BSAeq) | 0.066 ± 0.003 | 0.080 ± 0.001 |
| KM (mM) | 0.160 ± 0.015 | 0.075 ± 0.005 |
| kcat (min−1) | 8.38 × 102 ± 0.003 | 1.02 × 103 ± 0.001 |
| Ka (min−1 mM−1) | 5.24 × 103 ± 49.12 | 1.35 × 104 ± 910.7 |
| R2 | 0.96 | 0.99 |
| Protease Dosage (μL/L) | K (g/h) | M (1/h) |
|---|---|---|
| Ctrl | 0.0041 (0.0034–0.0043) | 169.9 (160.8–179.0) |
| 2 | 0.0038 (0.0031–0.0042) | 178.6 (169.0–188.2) |
| 5 | 0.0036 (0.0032–0.0041) | 192.9 (169.5–203.3) |
| 10 | 0.0041 (0.0034–0.0043) | 168.8 (159.7–179.6) |
| 30 | 0.0039 (0.0034–0.0041) | 176.4 (166.9–185.9) |
| 50 | 0.0047 (0.0044–0.0049) | 146.7 (144.1–149.3) |
| 70 | 0.0053 (0.0049–0.0058) | 129.9 (127.8–132.3) |
| 100 | 0.0091 (0.0087–0.0093) | 76.24 (74.9–79.6) |
| 150 | 0.0090 (0.0086–0.0094) | 76.20 (74.6–79.8) |
| Protease Dosage (µL/L) | L* | h* | C* | ΔE |
|---|---|---|---|---|
| Ctrl | 95.5 (95.1–95.9) | 81.1 (79.6–82.5) | 9.3 (8.9–10.8) | - |
| 2 | 95.3 (94.9–95.7) | 81.2 (79.7–82.6) | 9.0 (8.8–9.2) | 0.27 (0.29–0.31) |
| 5 | 95.3 (94.7–95.9) | 81.4 (79.9–82.8) | 8.9 (8.7–9.1) | 0.30 (0.28–0.31) |
| 10 | 95.3 (94.6–95.8) | 81.7 (80.2–83.1) | 8.9 (8.8–9.3) | 0.40 (0.39–0.41) |
| 30 | 96.4 (96.0–96.8) | 81.7 (80.3–83.3) | 8.9 (8.6–9.1) | 0.91 (0.87–0.93) |
| 50 | 95.4 (95.0–95.8) | 81.7 (80.1–82.9) | 9.0 (8.8–9.2) | 0.93 (0.91–0.95) |
| 70 | 96.8 (96.4–97.2) | 82.2 (80.7–83.6) | 10.6 (10.4–10.8) | 1.2 (1.0–1.4) |
| 100 | 96.4 (95.9–96.9) | 82.4 (80.9–83.9) | 10.6 (10.3–10.7) | 1.0 (0.8–1.3) |
| 150 | 96.5 (96.1–96.9) | 81.9 (80.4–83.4) | 10.4 (10.2–10.6) | 1.3 (1.0–1.4) |
| L* | h* | C* | ΔE | |||||
|---|---|---|---|---|---|---|---|---|
| Protease Dosage (µL/L) | Middle AF | End AF | Middle AF | End AF | Middle AF | End AF | Middle AF | End AF |
| Ctrl | 98.5 (98.1–98.9) | 95.4 (95.0–95.8) | 83.6 (82.1–85.1) | 84.8 (83.3–86.3) | 8.9 (8.7–10.1) | 8.5 (7.9–9.3) | - | - |
| 2 | 98.1 (97.7–98.5) | 95.2 (94.8–95.6) | 81.8 (80.3–83.3) | 81.8 (80.1–83.5) | 8.8 (8.6–9.0) | 8.2 (8.0–8.4) | 2.8 (2.6–2.9) | 3.4 (3.2–3.6) |
| 5 | 97.4 (97.0–97.8) | 95.2 (94.7–95.7) | 82.3 (80.8–83.8) | 82.1 (80.6–83.6) | 9.0 (8.8–9.2) | 8.1 (7.9–8.3) | 2.8 (2.5–2.9) | 3.4 (3.2–3.5) |
| 10 | 97.8 (97.4–98.2) | 95.3 (94.9–95.7) | 85.9 (84.4–87.4) | 81.3 (79.9–82.8) | 8.9 (8.5–9.0) | 7.9 (7.7–8.2) | 2.7 (2.5–2.8) | 3.3 (3.1–3.5) |
| 30 | 98.1 (97.6–98.6) | 95.2 (94.6–95.7) | 85.9 (84.5–87.7) | 82.2 (80.7–83.7) | 9.1 (8.9–9.3) | 8.3 (8.0–8.6) | 2.6 (2.4–2.7) | 3.3 (3.2–3.5) |
| 50 | 97.9 (97.5–98.3) | 95.2 (94.7–95.6) | 85.1 (83.6–86.6) | 81.8 (80.1–83.6) | 9.5 (9.3–9.7) | 8.3 (8.1–8.5) | 2.7 (2.6–2.9) | 3.2 (3.0–3.4) |
| 70 | 97.0 (96.6–97.4) | 94.9 (94.5–95.3) | 84.4 (82.9–85.9) | 83.2 (81.7–84.7) | 10.1 (9.9–10.3) | 9.1 (8.8–9.2) | 2.5 (2.4–2.7) | 3.2 (3.0–3.5) |
| 100 | 97.2 (96.8–97.6) | 94.9 (94.6–95.4) | 84.6 (83.1–86.1) | 83.3 (81.8–84.8) | 9.9 (9.6–10.5) | 9.2 (8.9–9.4) | 2.6 (2.3–2.7) | 3.3 (3.2–3.6) |
| 150 | 97.8 (97.4–98.2) | 95.0 (94.6–95.4) | 83.8 (82.3–85.3) | 83.1 (81.6–84.6) | 9.9 (9.7–10.1) | 8.9 (8.6–9.1) | 2.7 (2.5–2.8) | 3.4 (3.1–3.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benucci, I.; Lombardelli, C.; Muganu, M.; Mazzocchi, C.; Esti, M. A Minimally Invasive Approach for Preventing White Wine Protein Haze by Early Enzymatic Treatment. Foods 2022, 11, 2246. https://doi.org/10.3390/foods11152246
Benucci I, Lombardelli C, Muganu M, Mazzocchi C, Esti M. A Minimally Invasive Approach for Preventing White Wine Protein Haze by Early Enzymatic Treatment. Foods. 2022; 11(15):2246. https://doi.org/10.3390/foods11152246
Chicago/Turabian StyleBenucci, Ilaria, Claudio Lombardelli, Massimo Muganu, Caterina Mazzocchi, and Marco Esti. 2022. "A Minimally Invasive Approach for Preventing White Wine Protein Haze by Early Enzymatic Treatment" Foods 11, no. 15: 2246. https://doi.org/10.3390/foods11152246
APA StyleBenucci, I., Lombardelli, C., Muganu, M., Mazzocchi, C., & Esti, M. (2022). A Minimally Invasive Approach for Preventing White Wine Protein Haze by Early Enzymatic Treatment. Foods, 11(15), 2246. https://doi.org/10.3390/foods11152246