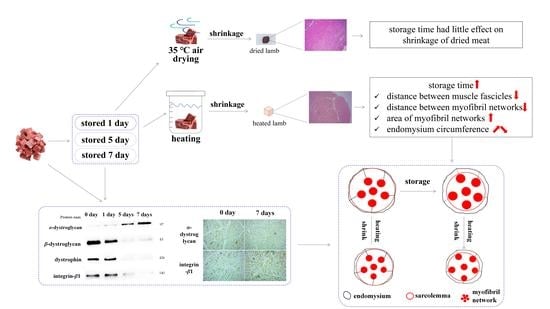
Shrinkage Properties and Their Relationship with Degradation of Proteins Linking the Endomysium and Myofibril in Lamb Meat Submitted to Heating or Air Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Heating, Air Drying, and Calculation of Shrinkage Ratio of Lamb Meat
2.3. Microstructure Measurement
2.4. Western Blotting
2.5. Activity of Matrix Metalloproteinases (MMPs)
2.6. Immunohistochemistry Analysis
2.7. Statistical Analysis
3. Results
3.1. Shrinkage of Lamb Meat after Heat Treatment at Different Temperatures
3.2. Shrinkage of Lamb Meat after Air-Drying Treatment
3.3. Changes in the Microstructure of Lamb Meat after Heat or Air-Drying Treatment
3.4. Degradation of Proteins Linking Endomysium and Myofibril during Storage
4. Discussion
4.1. The Effect of Postmortem Aging and Heating on Longitudinal, Transverse and Volume Shrinkage of Lamb Meat Cubes
4.2. Effect of Storage Aging and Heating on Porosity of Lamb Meat Cubes
4.3. The Effect of Postmortem Aging and Heating on Connective Tissue Shrinkage of Heated Lamb Meat Cubes
4.4. Effects of Degradation of Proteins Binding Endomysium and Myofibril Induced by Matrix Metalloproteinases (MMPs) on Gap Formation in Raw Lamb Meat
4.5. The Formation of Water Channels between Muscle Fascicles Might Play an Important Role in Shrinkage of Lamb Meat Cubes
4.6. Degradation of Protein Linking the Endomysium and Myofibril Did Not Have an Important Impact on Longitudinal, Transverse and Volume Shrinkage of 35 °C Air-Dried Lamb Meat Cubes Stored for 1 Day or Less Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Fito, P.J.; Balaguer, N. Thermodynamic model of meat drying by infrarred thermography. J. Food Eng. 2014, 128, 103–110. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent drying of food products: A critical review. J. Food Eng. 2014, 121, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, F.J.; Wiangkaew, C.; Pham, Q.T. Drying modeling and water diffusivity in beef meat. J. Food Eng. 2007, 78, 74–85. [Google Scholar] [CrossRef]
- Madiouli, J.; Sghaier, J.; Lecomte, D.; Sammouda, H. Determination of porosity change from shrinkage curves during drying of food material. Food Bioprod. Process. 2012, 90, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- May, B.K.; Perré, P. The importance of considering exchange surface area reduction to exhibit a constant drying flux period in foodstuffs. J. Food Eng. 2002, 54, 271–282. [Google Scholar] [CrossRef]
- Liu, J.; Ellies-Oury, M.; Chriki, S.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquettea, J. Contributions of tenderness, juiciness and flavor liking to overall liking of beef in Europe. Meat Sci. 2020, 168, 108190. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, ageing and temperature influence the changes in texture, cooking loss and shrinkage of cooked beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Purslow, P.P. Effect of postrigor sarcomere length on mechanical and structural characteristics of raw and heat-denatured single porcine muscle fibres. J. Texture Stud. 1996, 27, 217–233. [Google Scholar] [CrossRef]
- Mutungi, G.; Purslow, P.P.; Warkup, C. Structural and mechanical changes in raw and cooked single porcine muscle fibres extended to fracture. Meat Sci. 1995, 40, 217–234. [Google Scholar] [CrossRef]
- Purslow, P.P. Hydrothermal isometric tension properties of perimysial connective tissue in bovine semitendinosus muscle. In Proceedings of the 60th International Congress of Meat Science and Technology, Punte del Este, Uruguay, 17–22 August 2014. [Google Scholar]
- Latorre, M.E.; Velázquez, D.E.; Purslow, P.P. Differences in the energetics of collagen denaturation in connective tissue from two muscles. Int. J. Biol. Macromol. 2018, 113, 1294–1301. [Google Scholar] [CrossRef]
- Latorre, M.E.; Velázquez, D.E.; Purslow, P.P. The thermal shrinkage force in perimysium from different beef muscles is not affected by post-mortem ageing. Meat Sci. 2018, 135, 109–114. [Google Scholar] [CrossRef]
- Champion, A.E.; Purslow, P.P.; Duance, V.C. Dimensional changes of isolated endomysia on heating. Meat Sci. 1988, 24, 261–273. [Google Scholar] [CrossRef]
- Christensen, M.; Purslow, P.P.; Larsen, L.M. The effect of cooking temperature on mechanical properties of whole meat, single muscle fibres and perimysial connective tissue. Meat Sci. 2000, 55, 301–307. [Google Scholar] [CrossRef]
- Purslow, P.P.; Trotter, J.A. The morphology and mechanical properties of endomysium in series-fibred muscles: Variations with muscle length. J. Muscle Res. Cell Motil. 1994, 15, 299–308. [Google Scholar] [CrossRef]
- Lewis, G.J.; Purslow, P.P. The strength and stiffness of perimysial connective tissue isolated from cooked beef muscle. Meat Sci. 1989, 26, 255–269. [Google Scholar] [CrossRef]
- Lewis, G.J.; Purslow, P.P.; Rice, A.E. The effect of conditioning on the strength of perimysial connective tissue dissected from cooked meat. Meat Sci. 1991, 30, 1–12. [Google Scholar] [CrossRef]
- Omairi, S.; Hau, K.L.; Collins-Hooper, H.; Scott, C.; Vaiyapuri, S.; Torelli, S.; Montanaro, F.; Matsakas, A.; Patel, K. Regulation of the dystrophin associated glycoprotein complex composition by the metabolic properties of muscle fibres. Sci. Rep. 2019, 9, 2770–2781. [Google Scholar] [CrossRef] [Green Version]
- Sbardella, D.; Sciandra, F.; Gioia, M.; Marini, S.; Gori, A.; Giardina, B.; Tarantino, U.; Coletta, M.; Brancaccio, A.; Bozzie, M. α-dystroglycan is a potential target of matrix metalloproteinase MMP-2. Matrix Biol. 2015, 41, 2–7. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, X.; Chen, H.; Zhong, D.; Jin, J.; Qin, Q.; Zhang, H.; Ma, S.; Li, G. β-Dystroglycan cleavage by matrix metalloproteinase-2/-9 disturbs aquaporin-4 polarization and influences brain edema in acute cerebral ischemia. Neuroscience 2016, 326, 141–157. [Google Scholar] [CrossRef]
- Sbardella, D.; Inzitari, R.; Iavarone, F.; Gioia, M.; Marini, S.; Sciandra, F.; Castagnola, M.; Van den Steen, P.E.; Opdenakker, G.; Giardina, B.; et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse β-dystroglycan. Iubmb Life 2012, 64, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Court, F.A.; Zambroni, D.; Pavoni, E.; Colombelli, C.; Baragli, C.; Figlia, G.; Sorokin, L.; Ching, W.; Salzer, J.L.; Wrabetz, L.; et al. MMP2-9 cleavage of dystroglycan alters the size and molecular composition of schwann cell domains. J. Neurosci. 2011, 31, 12208–12217. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Singh, M.; Ross, R.S.; Johnson, J.N.; Singh, K. Beta-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of beta1 integrin signaling and mitochondrial pathway. Am. J. Physiol. Cell Physiol. 2006, 290, C254–C261. [Google Scholar] [CrossRef] [PubMed]
- Veiseth-Kent, E.; Pedersen, M.E.; Rønning, S.B.; Rødbotten, R. Can postmortem proteolysis explain tenderness differences in various bovine muscles? Meat Sci. 2017, 137, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astruc, T.; Gatellier, P.; Labas, R.; Lhoutellier, V.S.; Marinova, P. Microstructural changes in m. rectus abdominis bovine muscle after heating. Meat Sci. 2010, 85, 743–751. [Google Scholar] [CrossRef]
- Supaphon, P.; Kerdpiboon, S.; Vénien, A.; Loison, O.; Sicard, J.; Rouel, J.; Astruc, T. Structural changes in local Thai beef during sous-vide cooking. Meat Sci. 2021, 175, 108442. [Google Scholar] [CrossRef]
- Porcari, P.; Hall, M.G.; Clark, C.A.; Greally, E.; Straub, V.; Blamire, A.M. The effects of ageing on mouse muscle microstructure: A comparative study of time-dependent diffusion MRI and histological assessment. NMR Biomed. 2018, 31, e3881. [Google Scholar] [CrossRef]
- Allikian, M.J.; McNally, E.M. Processing and assembly of the dystrophin glycoprotein complex. Traffic 2007, 8, 177–183. [Google Scholar] [CrossRef]
- Martin, P.T. Dystroglycan glycosylation and its role in matrix binding in skeletal muscle. Glycobiology 2003, 13, 55r–66r. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Ertbjerg, P. Sarcoplasmic and myofibril-bound calpains during storage of pork longissimus muscle: New insights on protein degradation. Food Chem. 2022, 372, 131347. [Google Scholar] [CrossRef]
- Calkins, C.R.; Seideman, S.C. Relationships among calcium-dependent protease, cathepsins B and H, meat tenderness and the response of muscle to aging. J. Anim. Sci. 1988, 66, 1186–1193. [Google Scholar] [CrossRef]
- Purslow, P.P.; Oiseth, S.; Hughes, J.; Warner, R.D. The structural basis of cooking loss in beef: Variations with temperature and ageing. Food Res. Int. 2016, 89, 739–748. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R.D. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef]
- Herbage, D.; Lucas, J.M.; Huc, A. Collagen and proteoglycan interactions in bovine articular cartilage. Biochim. Biophys. Acta 1974, 336, 108–116. [Google Scholar] [CrossRef]
- Latorre, M.E.; Velazquez, E. Effects of thermal treatment on collagen present in bovine M. Semitendinosus intramuscular connective tissue. Analysis of the chemical, thermal and mechanical properties. Food Struct. 2020, 27, 100165. [Google Scholar] [CrossRef]
- Bendall, J.R.; Restall, D.J. The cooking of single myofibres, small myofibre bundles and muscle strips from beef M. psoas and M. sternomandibularis muscles at varying heating rates and temperatures. Meat Sci. 1983, 8, 93–117. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Ong, L.; Kearney, G.; White, J.D.; Gras, S.; Warner, R.D. Ageing and cathepsin inhibition affect the shrinkage of fibre fragments of bovine semitendinosus, biceps femoris and psoas major during heating. Meat Sci. 2020, 172, 108339. [Google Scholar] [CrossRef]
- Locker, R.H.; Daines, G.J. Effect of shortening during cooking on the tenderness and histology of beef. J. Sci. Food Agric. 1975, 26, 1711–1720. [Google Scholar] [CrossRef]
- Barbera, S.; Tassone, S. Meat cooking shrinkage: Measurement of a new meat quality parameter. Meat Sci. 2006, 73, 467–474. [Google Scholar] [CrossRef]
- Aktaş, N.; Kaya, M. Influence of weak organic acids and salts on the denaturation characteristics of intramuscular connective tissue. A differential scanning calorimetry study. Meat Sci. 2001, 58, 413–419. [Google Scholar] [CrossRef]
- Rochdi, A.; Foucat, L.; Renou, J.P. NMR and DSC studies during thermal denaturation of collagen. Food Chem. 2000, 69, 295–299. [Google Scholar] [CrossRef]
- Voutila, L.; Mullen, A.M.; Ruusunen, M.; Troy, D.; Puolanne, E. Thermal stability of connective tissue from porcine muscles. Meat Sci. 2007, 76, 474–480. [Google Scholar] [CrossRef]
- Kijowski, J.M.; Mast, M.G. Thermal properties of proteins in chicken broiler tissues. J. Food Sci. 1988, 53, 363–366. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, Y.; Li, J.; Guo, X.; Cui, B.; Peng, Z. Contribution of cross-links and proteoglycans in intramuscular connective tissue to shear force in bovine muscle with different marbling levels and maturities. LWT 2016, 66, 413–419. [Google Scholar] [CrossRef]
- Allain, J.C.; Lous, M.L.; Bazin, S.; Bailey, A.J.; Delaunay, A. Isometric tension developed during heating of collagenous tissues. Relationships with collagen cross-linking. Biochim. Biophys. Acta 1978, 533, 147–155. [Google Scholar] [CrossRef]
- Nishiumi, T.; Fukuda, T.; Nishimura, T. Isolation and characterization of a small proteoglycan associated with porcine intramuscular connective tissue. J. Agric. Food Chem. 1997, 45, 2978–2983. [Google Scholar] [CrossRef]
- Snowden, J.M. The stabilization of in vivo assembled collagen fibrils by proteoglycans/glycosaminoglycans. Biochim. Biophys. Acta 1982, 703, 21–25. [Google Scholar] [CrossRef]
- Hannesson, K.O.; Pedersen, M.E.; Ofstad, R.; Kolset, S.O. Breakdown of large proteoglycans in bovine intramuscular connective tissue early postmortem. J. Muscle Foods 2003, 14, 301–318. [Google Scholar] [CrossRef]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, W.; Zhou, G.; Xu, X.; Peng, Z.; Hu, Z. Morphological and biochemical assessment of apoptosis in different skeletal muscles of bulls during conditioning. J. Anim. Sci. 2010, 88, 3439–3444. [Google Scholar] [CrossRef] [PubMed]
- Becila, S.; Herrera-Mendez, C.H.; Coulis, G.; Labas, R.; Astruc, T.; Picard, B.; Boudjellal, A.; Pelissier, P.; Bremaud, L.; Ouali, A. Postmortem muscle cells die through apoptosis. Eur. Food Res. Technol. 2010, 231, 485–493. [Google Scholar] [CrossRef]
- Foditsch, E.E.; Saenger, A.M.; Monticelli, F.C. Skeletal muscle proteins: A new approach to delimitate the time since death. Int. J. Legal Med. 2016, 130, 433–440. [Google Scholar] [CrossRef]
- Taylor, R.G.; Geesink, G.H.; Thompson, V.F.; Koohmaraie, M.; Goll, D.E. Is Z-disk degradation responsible for postmortem tenderization? J. Anim. Sci. 1995, 73, 1351–1367. [Google Scholar] [CrossRef] [Green Version]
- Kołczak, T.; Pospiech, E.; Palka, K.; Łącki, J. Changes in structure of psoas major and minor and semitendinosus muscles of calves, heifers and cows during post-mortem ageing. Meat Sci. 2003, 64, 77–83. [Google Scholar] [CrossRef]
- Wojtysiak, D.; Górska, M. Effect of aging time on meat quality and rate of desmin and dystrophin degradation of pale, soft, exudative (PSE) and normal turkey breast muscle. Folia Biol. 2018, 66, 63–72. [Google Scholar] [CrossRef]
- Krag, T.O.; Vissing, J. A new mouse model of limb-girdle muscular dystrophy type 2I homozygous for the common L276I mutation mimicking the mild phenotype in humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1137–1146. [Google Scholar]
- Hammers, D.W.; Sleeper, M.M.; Forbes, S.C.; Shima, A.; Walter, G.A.; Sweeney, H.L. Tadalafil treatment delays the onset of cardiomyopathy in dystrophin-deficient hearts. J. Am. Heart Assoc. 2016, 5, e003911. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Chen, T.; Li, H.; Li, Y.; Yang, R. Impact of postmortem degradation of cytoskeletal proteins on intracellular gap, drip channel and water-holding capacity. Meat Sci. 2021, 176, 108472. [Google Scholar] [CrossRef]
- Passerieux, E.; Rossignol, R.; Chopard, A.; Carnino, A.; Marini, J.F.; Letellier, T.; Delage, J.P. Structural organization of the perimysium in bovine skeletal muscle: Junctional plates and associated intracellular subdomains. J. Struct. Biol. 2006, 154, 206–216. [Google Scholar] [CrossRef]
- Passerieux, E.; Rossignol, R.; Letellier, T.; Delage, J.P. Physical continuity of the perimysium from myofibers to tendons: Involvement in lateral force transmission in skeletal muscle. J. Struct. Biol. 2007, 159, 19–28. [Google Scholar] [CrossRef]
- Wess, T.J.; Orgel, J.P. Changes in collagen structure: Drying, dehydrothermal treatment and relation to long term deterioration. Thermochim. Acta 2000, 365, 119–128. [Google Scholar] [CrossRef]




| Storage Time | Heating Temperature | p Value | |||||
|---|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 90 °C | Storage Time | Heating Temperature | Storage Time × Heating Temperature | ||
| Longitudinal-shrinkage ratio (%) | 1 day | −0.31 ± 3.60 Ac | 6.75 ± 1.68 Bb | 25.95 ± 4.52 Aa | 0.006 | <0.001 | 0.600 |
| 5 days | 0.55 ± 4.33 Ac | 13.71 ± 4.16 Ab | 30.27 ± 2.32 Aa | ||||
| 7 days | 4.30 ± 2.42 Ac | 13.29 ± 3.14 Ab | 31.38 ± 0.83 Aa | ||||
| Transverse-shrinkage ratio (%) | 1 day | 8.69 ± 5.30 Ab | 24.59 ± 4.49 Aa | 28.20 ± 6.11 Aa | 0.001 | <0.001 | 0.892 |
| 5 days | 4.80 ± 2.28 Ab | 22.88 ± 4.43 ABa | 23.78 ± 3.20 ABa | ||||
| 7 days | 2.13 ± 2.89 Ab | 16.50 ± 0.56 Ba | 18.56 ± 1.78 Ba | ||||
| Volume shrinkage ratio (%) | 1 day | 6.47 ± 4.49 c | 39.60 ± 5.17 b | 53.46 ± 0.43 a | 0.967 | <0.001 | 0.427 |
| 5 days | 3.05 ± 17.40 c | 41.38 ± 8.64 b | 53.95 ± 4.97 a | ||||
| 7 days | 10.36 ± 5.40 c | 38.08 ± 3.03 b | 52.40 ± 3.75 a | ||||
| 1 Day | 5 Days | 7 Days | p Value | |
|---|---|---|---|---|
| Longitudinal-shrinkage ratio (%) | 94.70 ± 3.56 | 90.17 ± 6.84 | 88.77 ± 4.25 | 0.387 |
| Transverse-shrinkage ratio (%) | 78.73 ± 9.89 | 77.88 ± 2.55 | 85.10 ± 4.76 | 0.390 |
| Volume shrinkage (%) | 54.51 ± 5.79 | 58.93 ± 6.91 | 59.97 ± 5.83 | 0.553 |
| 1 Day | 5 Days | 7 Days | p Value | |
|---|---|---|---|---|
| Raw lamb muscle | 5.31 ± 3.22 c | 9.23 ± 3.30 b | 13.67 ± 7.25 a | 0.012 |
| Parameter | Treatment (Heating or Air Drying) | Storage Time | p Value | ||||
|---|---|---|---|---|---|---|---|
| 1 Day | 5 Days | 7 Days | Storage Time | Treatment | Storage Time× Treatment | ||
| Distance between muscle bundles (μm) | Raw meat | 4.88 ± 1.91 Aa | 4.15 ± 1.23 Ab | 3.70 ± 2.22 Ac | <0.001 | 0.002 | 0.424 |
| Outside heated meat | 3.71 ± 1.23 Ca | 3.49 ± 1.88 Ba | 2.59 ± 1.00 Cb | ||||
| Inside heated meat | 4.35 ± 1.64 Ba | 3.51 ± 1.48 Bb | 2.99 ± 1.38 Bc | ||||
| Raw meat | 4.88 ± 1.91 Aa | 4.15 ± 1.23 Bb | 3.70 ± 2.22 Bb | <0.001 | <0.001 | 0.074 | |
| Outside dried meat | 2.29 ± 1.09 Cb | 2.54 ± 1.19 Cb | 3.43 ± 2.95 Ba | ||||
| Inside dried meat | 4.00 ± 2.61 Bc | 4.65 ± 2.56 Ab | 6.34 ± 5.84 Aa | ||||
| Lamb meat Porosity (%) | Raw meat | 49.07 ± 4.91 a | 45.19 ± 9.30 b | 37.16 ± 9.56 c | <0.001 | <0.001 | 0.005 |
| Outside heated meat | 19.72 ± 5.85 g | 28.21 ± 21.14 e | 22.58 ± 5.45 f | ||||
| Inside heated meat | 29.38 ± 3.60 e | 33.54 ± 15.23 d | 27.86 ± 3.33 ef | ||||
| Raw meat | 49.07 ± 4.91 a | 45.19 ± 9.30 b | 37.16 ± 9.56 c | 0.058 | <0.001 | 0.008 | |
| Outside dried meat | 17.04 ± 8.86 d | 20.28 ± 9.66 d | 18.62 ± 9.74 d | ||||
| Inside dried meat | 36.92 ± 13.92 c | 45.05 ± 8.55 b | 38.33 ± 12.64 c | ||||
| Distance between myofibril networks (μm) | Raw meat | 2.23 ± 0.64 Aa | 2.15 ± 0.64 Aa | 1.75 ± 1.02 Ab | 0.001 | <0.001 | 0.940 |
| Outside heated meat | 1.41 ± 0.70 Ba | 1.26 ± 0.56 Bb | 0.88 ± 0.49 Bc | ||||
| Inside heated meat | 1.49 ± 0.71 Ba | 1.17 ± 0.51 Bb | 0.92 ± 0.41 Bc | ||||
| Raw meat | 2.23 ± 0.64 A | 2.15 ± 0.64 A | 1.75 ± 1.02 A | 0.104 | <0.001 | 0.474 | |
| Outside dried meat | 0.88 ± 0.31 C | 0.84 ± 0.32 C | 0.78 ± 0.47 C | ||||
| Inside dried meat | 1.45 ± 0.72 B | 1.73 ± 0.58 B | 1.39 ± 0.83 B | ||||
| Area of myofibril networks (μm2) | Raw meat | 65.03 ± 17.98 Ab | 64.14 ± 21.57 Ab | 86.72 ± 32.21 Aa | 0.019 | <0.001 | 0.101 |
| Outside heated meat | 53.45 ± 16.52 Ba | 48.31 ± 16.91 Cb | 53.01 ± 16.59 Ca | ||||
| Inside heated meat | 52.92 ± 14.07 Bb | 57.04 ± 19.19 Bab | 58.88 ± 14.31 Ba | ||||
| Raw meat | 65.03 ± 17.98 d | 64.14 ± 21.57 d | 86.72 ± 32.21 a | 0.179 | <0.001 | <0.001 | |
| Outside dried meat | 61.89 ± 15.74 de | 59.31 ± 19.04 e | 45.61 ± 13.13 f | ||||
| Inside dried meat | 74.81 ± 15.17 c | 79.57 ± 47.36 b | 65.69 ± 19.02 d | ||||
| Endomysium circumference of muscle (μm) | Raw meat | 31.81 ± 4.23 Aa | 32.18 ± 4.43 Aa | 31.41 ± 4.90 Aa | <0.001 | <0.001 | 0.449 |
| Outside heated meat | 27.32 ± 2.86 Bb | 29.61 ± 3.22 Ba | 29.27 ± 4.90 Ca | ||||
| Inside heated meat | 27.49 ± 3.93 Bb | 30.41 ± 4.87 Ba | 30.15 ± 5.53 Ba | ||||
| Raw meat | 31.81 ± 4.23 ab | 32.18 ± 4.43 ab | 31.41 ± 4.90 b | <0.001 | <0.001 | <0.001 | |
| Outside dried meat | 25.71 ± 2.97 e | 26.82 ± 4.78 d | 24.87 ± 3.34 e | ||||
| Inside dried meat | 29.31 ± 2.98 c | 32.84 ± 4.56 a | 29.02 ± 3.97 c | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, W.; Shi, Z.; Liu, S.; Shu, Y.; Chai, X.; Zhang, Z. Shrinkage Properties and Their Relationship with Degradation of Proteins Linking the Endomysium and Myofibril in Lamb Meat Submitted to Heating or Air Drying. Foods 2022, 11, 2242. https://doi.org/10.3390/foods11152242
Rao W, Shi Z, Liu S, Shu Y, Chai X, Zhang Z. Shrinkage Properties and Their Relationship with Degradation of Proteins Linking the Endomysium and Myofibril in Lamb Meat Submitted to Heating or Air Drying. Foods. 2022; 11(15):2242. https://doi.org/10.3390/foods11152242
Chicago/Turabian StyleRao, Weili, Zhenxiao Shi, Sijia Liu, Ying Shu, Xiaoyu Chai, and Zhisheng Zhang. 2022. "Shrinkage Properties and Their Relationship with Degradation of Proteins Linking the Endomysium and Myofibril in Lamb Meat Submitted to Heating or Air Drying" Foods 11, no. 15: 2242. https://doi.org/10.3390/foods11152242
APA StyleRao, W., Shi, Z., Liu, S., Shu, Y., Chai, X., & Zhang, Z. (2022). Shrinkage Properties and Their Relationship with Degradation of Proteins Linking the Endomysium and Myofibril in Lamb Meat Submitted to Heating or Air Drying. Foods, 11(15), 2242. https://doi.org/10.3390/foods11152242