A Novel Ethanolic Two-Phase System Based on Deep Eutectic Solvents and Amphiphilic Copolymer for the Extraction of Neohesperidin and Naringin from the Pomelo Peel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
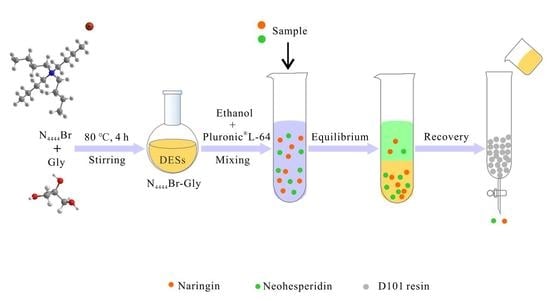
2.2. Preparation of DESs
2.3. Determination of the Phase Diagrams
2.4. Extraction of Nar and Neo Using DESs/PL 64 ETPSs
2.5. Recovery of Nar and Neo from DESs
2.6. HPLC Analysis
3. Results and Discussion
3.1. The Phase Behavior of DESs/PL 64 ETPS
3.2. Extracting of Nar and Neo in Different DESs/PL 64 ETPSs
3.2.1. Effect of the DESs types
3.2.2. Effect of the Alkyl Chain Length for DESs
3.3. Single-Factor Experiments
3.3.1. Effect of the Mass Ratio of DESs and PL 64
3.3.2. Effect of the Concentration of DESs
3.3.3. Effect of the Concentration of PL 64
3.3.4. Effect of the Molar Ratio of HBAs and HBDs
3.3.5. Effect of the Equilibrium Temperature
3.3.6. Effect of the Phosphate Buffer Solutions
3.3.7. Effect of the Ethanolic pH
3.4. Extraction of Nar and Neo from the Pomelo Peel
3.5. Recovery of Nar and Neo from DESs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- Guo, C.X.; Xu, F.; Zang, J.; Chen, D.Z.; Zhang, T.; Zhan, H.; Lu, M.; Zhuge, H.X. Neohesperidin induces cellular apoptosis in human breast adenocarcinoma MDA-MB-231 cells via activating the Bcl-2/Bax-mediated signaling pathway. Nat. Prod. Commun. 2012, 7, 1475–1478. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Guan, X.; Zhou, Z. The anti-aging potential of neohesperidin and its synergistic effects with other citrus flavonoids in extending chronological lifespan of Saccharomyces Cerevisiae BY4742. Molecules 2019, 24, 4093. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NFkappaB/COX2caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef]
- Robertson, G.H.; Clark, J.P.; Lundin, R. Dihydrochalcone sweeteners: Preparation of neohesperidin dihydrochalcone. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 125–129. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Xu, J.J.; Yang, R.; Ye, L.H.; Cao, J.; Cao, W.; Hu, S.S.; Peng, L.Q. Application of ionic liquids for elution of bioactive flavonoid glycosides from lime fruit by miniaturized matrix solid-phase dispersion. Food Chem. 2016, 204, 167–175. [Google Scholar] [CrossRef]
- Mitani, R.; Tashiro, H.; Arita, E.; Ono, K.; Haraguchi, M.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Extraction of nobiletin and tangeretin with antioxidant activity from peels of Citrus poonensis using liquid carbon dioxide and ethanol entrainer. Sep. Sci. Technol. 2021, 56, 290–300. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Vilet, N.Z.; Gue, E.; Servent, A.; Delalonde, M.; Wisniewski, C. Filtration-compression step as downstream process for flavonoids extraction from citrus peels: Performances and flavonoids dispersion state in the filtrate. Food Bioprod. Process. 2020, 120, 104–113. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, L.L.; Tan, Z.J. Polarity-controlled deep eutectic solvents-based biphasic system for the selective separation of geniposidic acid and aucubin from Eucommia ulmoides male flowers. J. Mol. Liq. 2022, 358, 119200. [Google Scholar] [CrossRef]
- Tang, W.; An, Y.; Row, K.H. Emerging applications of (micro) extraction phase from hydrophilic to hydrophobic deep eutectic solvents: Opportunities and trends. TrAC-Trend. Anal. Chem. 2021, 136, 116187. [Google Scholar] [CrossRef]
- Chabib, C.M.; Ali, J.K.; Jaoude, M.A.; Alhseinat, E.; Adeyemi, I.A.; Al Nashef, I.M. Application of deep eutectic solvents in water treatment processes: A review. J. Water. Process Eng. 2022, 47, 102663. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 12049. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, L.J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z.J. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Wang, M.; Bi, W.; Li, H.; Chen, D.D. High-throughput analysis for Artemisinins with deep eutectic solvents mechanochemical extraction and direct analysis in real time mass spectrometry. Anal. Chem. 2018, 90, 3109–3117. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, S.S.; Liu, L.L. Recovery of natural active molecules using aqueous two-phase systems comprising of ionic liquids/deep eutectic solvents. Green Chem. Eng. 2022, 3, 5–14. [Google Scholar] [CrossRef]
- Shang, X.C.; Zhang, Y.Q.; Zheng, Y.F.; Li, Y.Q. Temperature-responsive deep eutectic solvents as eco-friendly and recyclable media for microwave extraction of flavonoid compounds from waste onion (Allium cepa L.) skins. Biomass Convers. Biorefin. 2022, 274, 123047. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Air assisted dispersive liquid-liquid microextraction (AA-DLLME) using hydrophilic-hydrophobic deep eutectic solvents for the isolation of monosaccharides and amino acids from kelp. Anal. Lett. 2020, 53, 188–202. [Google Scholar] [CrossRef]
- Buarque, F.S.; Soares, C.M.F.; De Souza, R.L.; Pereira, M.M.; Lima, A.S. Development of an ethanolic two-phase system (ETPS) based on polypropylene glycol 2000+ethylene glycol plus ethanol for separation of hydrophobic compounds. Chem. Commun. 2021, 57, 2156–2159. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Bahadur, P. Aggregation of water-soluble block copolymers in aqueous solutions: Recent trends. Adv. Colloid Interface Sci. 2006, 123–126, 75–96. [Google Scholar] [CrossRef]
- Shiran, H.S.; Baghbanbashi, M.; Ahsaie, F.G.; Pazuki, G. Study of curcumin partitioning in polymer-salt aqueous two phase systems. J. Mol. Liq. 2020, 303, 112629. [Google Scholar] [CrossRef]
- Camelo, L.C.A.; Santos, G.D.D.; De Souza, R.L.; Soares, C.M.F.; Pereira, J.F.B.; Lima, A.S. Pre-purification of genipin from genipap using aqueous-two-phase systems composed of protic ionic liquids plus polymers + water at 298 K and atmospheric pressure. Sep. Purif. Technol. 2021, 256, 117843. [Google Scholar] [CrossRef]
- Gao, C.; Cai, C.Y.; Liu, J.J.; Wang, Y.N.; Chen, Y.Z.; Wang, L.Q.; Tan, Z.J. Extraction and preliminary purification of polysaccharides from Camellia oleifera Abel. seed cake using a thermoseparating aqueous two-phase system based on EOPO copolymer and deep eutectic solvents. Food Chem. 2020, 313, 126164. [Google Scholar] [CrossRef]
- Silva, F.A.E.; Carmo, R.M.C.; Fernandes, A.P.M.; Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Using ionic liquids to tune the performance of aqueous biphasic systems based on Pluronic L-35 for the purification of naringin and rutin. ACS Sustain. Chem. Eng. 2017, 5, 6409–6419. [Google Scholar] [CrossRef]
- Kurnik, I.S.; Noronha, M.A.; Camara, M.C.C.; Mazzola, P.G.; Vicente, A.A.; Pereira, J.F.B.; Lopes, A.M. Separation and purification of curcumin using novel aqueous two-phase micellar systems composed of amphiphilic copolymer and cholinium ionic liquids. Sep. Purif. Technol. 2020, 250, 117262. [Google Scholar] [CrossRef]
- Ahsaie, F.G.; Pazuki, G. Effect of carbohydrates, choline chloride based deep eutectic solvents and salts on the phase behavior of PEG-PPG copolymer ATPSs and partitioning of penicillin G. J. Mol. Liq. 2021, 339, 117152. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sen, K. Ionic liquid vs tri-block copolymer in a new aqueous biphasic system for extraction of Zn-cholesterol complex. J. Mol. Liq. 2017, 229, 278–284. [Google Scholar] [CrossRef]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation studies on phase inversion. J. Chromatogr. B 1998, 711, 285–293. [Google Scholar] [CrossRef]
- Moradi, F.; Shahrouzi, J.R. Phase equilibrium and partitioning of cephalosporins (cephalexin, cefazolin, cefixime) in aqueous two-phase systems based on carbohydrates (glucose, fructose, sucrose, maltose)/acetonitrile. Fluid Phase Equilib. 2020, 507, 112388. [Google Scholar] [CrossRef]
- Guo, W.; Hou, Y.; Wu, W.; Ren, S.; Tian, S.; Marsh, K.N. Separation of phenol from model oils with quaternary ammonium saltsvia forming deep eutectic solvents. Green Chem. 2013, 15, 226–229. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]





| Abbreviations | HBAs | HBDs | Water | Mole Ratios | pH | Densities (g/cm3) |
|---|---|---|---|---|---|---|
| ChCl-Mal | Choline chloride | D-(+)-Maltose monohydrate | H2O | 3:1:3 | 5.04 | 1.2387 |
| ChCl-Glu | Choline chloride | D-(+)-Glucose | H2O | 2:1:3 | 5.92 | 1.2206 |
| ChCl-Gly | Choline chloride | Glycerol | 1:2 | 7.45 | 1.1912 | |
| ChCl-OA | Choline chloride | Oxalic acid | 1:1 | 1.00 | 1.2193 | |
| Bet-Gly | Betaine | Glycerol | 1:2 | 8.73 | 1.2283 | |
| Pro-Gly | L-(-)-Proline | Glycerol | 2:5 | 7.60 | 1.2757 | |
| Pro-Xyl | L-(-)-Proline | Xylitol | H2O | 1:1:5 | 6.73 | 1.2648 |
| N2222Cl-Glu | Tetraethylammonium chloride | D-(+)-Glucose | H2O | 2:1:3 | 5.42 | 1.1321 |
| N2222Cl-Gly | Tetraethylammonium chloride | Glycerol | 1:1 | 7.60 | 1.0385 | |
| N4444Cl-Gly | Tetrabutylammonium chloride | Glycerol | 1:2 | 5.11 | 1.0394 | |
| N2222Br-Gly | Tetraethlammonium bromide | Glycerol | 1:2 | 10.14 | 1.2315 | |
| N3333Br-Gly | Tetrapropylammonium bromide | Glycerol | 1:2 | 5.97 | 1.1650 | |
| N4444Br-Gly | Tetrabutylammonium bromide | Glycerol | 1:3 | 6.63 | 1.1307 | |
| 1:4 | 6.80 | 1.1407 | ||||
| 1:5 | 6.18 | 1.1516 | ||||
| 1:6 | 6.46 | 1.1679 | ||||
| 1:7 | 5.81 | 1.1644 |
| Parameters | N4444Br-Gly/PL 64 ETPSs (29 wt%:61 wt%) | |
|---|---|---|
| Standard Sample | Pomelo Peel Extract | |
| KNar | 6.66 ± 0.41 | 5.90 ± 0.55 |
| ENar | 31.68 ± 0.77% | 34.92 ± 3.11% |
| KNeo | 19.13 ± 0.90 | 6.87 ± 0.27 |
| ENeo | 13.91 ± 0.95% | 31.48 ± 1.77% |
| S | 2.88 ± 0.13 | 1.17 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Qin, Z.; Wang, Y.; Liu, L.; Tan, Z. A Novel Ethanolic Two-Phase System Based on Deep Eutectic Solvents and Amphiphilic Copolymer for the Extraction of Neohesperidin and Naringin from the Pomelo Peel. Foods 2022, 11, 2590. https://doi.org/10.3390/foods11172590
Wang S, Qin Z, Wang Y, Liu L, Tan Z. A Novel Ethanolic Two-Phase System Based on Deep Eutectic Solvents and Amphiphilic Copolymer for the Extraction of Neohesperidin and Naringin from the Pomelo Peel. Foods. 2022; 11(17):2590. https://doi.org/10.3390/foods11172590
Chicago/Turabian StyleWang, Shanshan, Zongkui Qin, Yicong Wang, Leilei Liu, and Zhijian Tan. 2022. "A Novel Ethanolic Two-Phase System Based on Deep Eutectic Solvents and Amphiphilic Copolymer for the Extraction of Neohesperidin and Naringin from the Pomelo Peel" Foods 11, no. 17: 2590. https://doi.org/10.3390/foods11172590
APA StyleWang, S., Qin, Z., Wang, Y., Liu, L., & Tan, Z. (2022). A Novel Ethanolic Two-Phase System Based on Deep Eutectic Solvents and Amphiphilic Copolymer for the Extraction of Neohesperidin and Naringin from the Pomelo Peel. Foods, 11(17), 2590. https://doi.org/10.3390/foods11172590