Curcumin-Loaded Self-Assembly Constructed by Octenylsuccinate Fish (Cyprinus carpio L.) Scale Gelatin: Preparation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
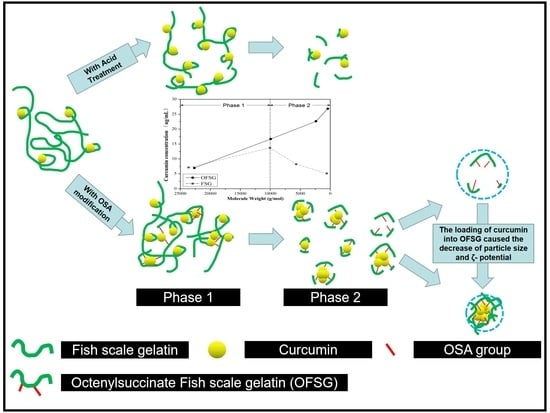
2.2. FSG Extraction and OFSG Synthesis
2.3. Determination of DS and Mw of OFSGs
2.4. Preparation of CL-FSG and CL-OFSG
2.5. Determination of Curcumin Concentration in CL-FSG or CL-OFSG
2.6. Characterization of FSG and OFSG with or without the Loading of Curcumin
2.7. Dynamic Light Scattering (DLS) and ζ-Potential
2.8. Transmission Electron Microscopy (TEM)
2.9. Curcumin Stability and Release of FSG and OFSG in Simulated Gastrointestinal Digestion
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Molecular Weight (Mw) and Degree of Substitution (DS) on the CLE (Curcumin Loading Efficiency, μg/mL) of OFSG
3.2. Characterization Analysis of FSG and OFSG with or without the Loading of Curcumin
3.3. Self-Assembly Observation of FSG and OFSG with or without the Loading of Curcumin
3.4. In-vitro Simulated Gastrointestinal Stability and Release of Curcumin-Loaded FSG and OFSG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Eun, J.-B.; Wierenga, P.A.; Gruppen, H. Antioxidative activity and emulsifying properties of cuttlefish skin gelatin modified by oxidised phenolic compounds. Food Chem. 2009, 117, 160–168. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.C.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Zou, Z.Z.; Shangguan, X.C.; Wang, H.; Bansal, N. Glycosylated fish gelatin emulsion: Rheological, tribological properties and its application as model coffee creamers. Food Hydrocoll. 2019, 102, 105552. [Google Scholar] [CrossRef]
- Xu, J.M.; Zhang, T.; Zhang, Y.Y.; Yang, L.L.; Nie, Y.H.; Tao, N.P.; Wang, X.C.; Zhong, J. Silver carp scale gelatins for the stabilization of fish oil-loaded emulsions. Int. J. Biol. Macromol. 2021, 186, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Bi, S.C.; You, X.G.; Wang, M.Y.; Cong, X.; Yuan, C.S.; Yu, M.; Cheng, X.J.; Chen, X.-G. Development and application of fish scale wastes as versatile natural biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Sow, L.C.; Tan, S.J.; Yang, H. Rheological properties and structure modification in liquid and gel of tilapia skin gelatin by the addition of low acyl gellan. Food Hydrocoll. 2019, 90, 9–18. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Sow, L.C.; Chong, J.M.N.; Liao, Q.X.; Yang, H. Effects of κ-carrageenan on the structure and rheological properties of fish gelatin. J. Food Eng. 2018, 239, 92–103. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Tang, W.; Wan, C.; Liu, J. Characterization of food gels prepared from the water extract of fish (Cyprinus carpio L.) scales: From molecular components to sensory attributes. Food Hydrocoll. 2021, 112, 106263. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.C.; Wang, H.; Zhang, N.H.; Zhang, L.; Sha, X.M. Gelation kinetics and characterization of enzymatically enhanced fish scale gelatin-pectin coacervate. J. Sci. Food Agric. 2018, 98, 1024–1032. [Google Scholar] [CrossRef]
- Wu, S.J.; Ho, Y.C.; Jiang, S.Z.; Mi, F.L. Effect of tannic acid-fish scale gelatin hydrolysate hybrid nanoparticles on intestinal barrier function and α-amylase activity. Food Funct. 2015, 6, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, W.; Lei, Z.; Wang, Z.; Liu, J. Effect of polysaccharides on the gel characteristics of “yu dong” formed with fish (Cyprinus carpio L.) scale aqueous extract. Food Chem. 2021, 338, 127792. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.S.; Basha, R.K.; Arifin, N.N.T.; Othman, S.H.; Mohammed, M.A.P. Functional properties of tilapia’s fish scale gelatin film: Effects of different type of plasticizers. Sains Malays. 2020, 49, 2221–2229. [Google Scholar] [CrossRef]
- Fu, B.; Mei, S.; Su, X.; Chen, H.; Zhu, J.; Zheng, Z.; Lin, H.; Dai, C.; Luque, R.; Yang, D. Integrating waste fish scale-derived gelatin and chitosan into edible nanocomposite film for perishable fruits. Int. J. Biol. Macromol. 2021, 191, 1164–1174. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Sha, X.M. Promotion of foam properties of egg white protein by subcritical water pre-treatment and fish scales gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 171–177. [Google Scholar] [CrossRef]
- Viji, P.; Phannendra, T.S.; Jesmi, D.; Rao, B.M.; Das, P.H.D.; George, N. Functional and antioxidant properties of gelatin hydrolysates prepared from skin and scale of sole fish. J. Aquat. Food Prod. Technol. 2019, 28, 976–986. [Google Scholar] [CrossRef]
- Mohtar, N.F.; Perera, C.O.; Hemar, Y. Chemical modification of new zealand hoki (Macruronus novaezelandiae) skin gelatin and its properties. Food Chem. 2014, 155, 64–73. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Huang, T.; Zhang, L.; Wang, G.Y.; Tu, Z.C. Microbial transglutaminase (MTGase) modified fish gelatin-γ-polyglutamic acid (γ-PGA): Rheological behavior, gelling properties, and structure. Food Chem. 2021, 348, 129093. [Google Scholar] [CrossRef]
- Leceta, I.; Urdanpilleta, M.; Zugasti, I.; Guerrero, P.; Caba, K.D.L. Assessment of gallic acid-modified fish gelatin formulations to optimize the mechanical performance of films. Int. J. Biol. Macromol. 2018, 120, 2131–2136. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, M.; Tao, L.; Liu, L.; Zhong, J. Octenyl succinic anhydride modification of bovine bone and fish skin gelatins and their application for fish oil-loaded emulsions. Food Hydrocoll. 2020, 108, 106041. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Sánchez, E.; Díaz-Calderón, P.; Blaker, J.J.; Enrione, J.; Quero, F. Synergistic effects of crosslinking and chitosan molecular weight on the microstructure, molecular mobility, thermal and sorption properties of porous chitosan/gelatin/hyaluronic acid scaffolds. J. Appl. Polym. Sci. 2017, 134, 44772. [Google Scholar] [CrossRef]
- Kulchaiyawat, C.; Wang, T.; Han, Z. Improving albumen thermal stability using succinylation reaction with octenyl succinic anhydride. LWT Food Sci. Technol. 2016, 73, 630–639. [Google Scholar] [CrossRef]
- Shah, N.N.; Umesh, K.V.; Singhal, R.S. Hydrophobically modified pea proteins: Synthesis, characterization and evaluation as emulsifiers in eggless cake. J. Food Eng. 2019, 255, 15–23. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ma, Y.; Chen, F.; Zhao, G. Synthesis, characterization, and aqueous self-assembly of octenylsuccinate oat β-glucan. J. Agric. Food Chem. 2013, 61, 12683–12691. [Google Scholar] [CrossRef]
- Qiu, D.; Bai, Y.J.; Shi, Y.C. Identification of isomers and determination of octenylsuccinate in modified starch by HPLC and mass spectrometry. Food Chem. 2012, 135, 665–671. [Google Scholar] [CrossRef]
- Castanha, N.; Divino, D.M.J.M.; Augusto, P.E.D. Potato starch modification using the ozone technology. Food Hydrocoll. 2017, 66, 343–356. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F.; Tian, W.; Ma, Y.; Li, J.; Zhao, G. Optimization and characterization of curcumin loaded in octenylsuccinate oat β-glucan micelles with an emphasis on degree of substitution and molecular weight. J. Agric. Food Chem. 2014, 62, 7532–7540. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.Z.; Li, X.T.; Zhang, N.; Tang, C.H. Novel soy β-conglycinin core-shell nanoparticles as outstanding ecofriendly nanocarriers for curcumin. J. Agric. Food Chem. 2019, 67, 6292–6301. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C. Structure and preparation of octenyl succinic esters of granular starch, microporous starch and soluble maltodextrin. Carbohydr. Polym. 2011, 83, 520–527. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.E.; Li, Z.; Liu, Q.; Zhao, X.; Yang, H. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019, 286, 87–97. [Google Scholar] [CrossRef]
- Liu, J.; Lei, L.; Ye, F.; Zhou, Y.; Younis, H.; Zhao, G. Aggregates of octenylsuccinate oat β-glucan as novel capsules to stabilize curcumin over food processing, storage and digestive fluids and to enhance its bioavailability. Food Funct. 2018, 9, 491–501. [Google Scholar] [CrossRef]
- Li, Y.C.; Liu, S.Y.; Meng, F.B.; Liu, D.Y.; Zhang, Y.; Wang, W.; Zhang, J.M. Comparative review and the recent progress in detection technologies of meat product adulteration. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2256–2296. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, Y.; Wu, B.; Hu, W.; He, M.; Hu, G. Novel mutagenesis and screening technologies for food microorganisms: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, J.; Liu, S.; Ding, Y.; Wang, W.; Zhou, X. A bottom-up evaluation on cryoprotective potentials of gelatine from fish scale. Food Hydrocoll. 2022, 124, 107243. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Processing 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.C.; Sha, X.M.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Petit, C.; Zemb, T.; Pileni, M.P. Structural study of microemulsion-based gels at the saturation point. Langmuir 1991, 7, 223–231. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010, 119, 669–674. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Wierenga, P.A.; Gruppen, H. Surface activity and molecular characteristics of cuttlefish skin gelatin modified by oxidized linoleic acid. Int. J. Biol. Macromol. 2011, 48, 650–660. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high-pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Baglole, K.N.; Boland, P.G.; Wagner, B.D. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A Chem. 2005, 173, 230–237. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Wang, H.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsifification properties of dodecenyl succinic anhydride derivatives of gum Arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Santacruz-Ortega, H.; Cinco-Moroyoqui, F.J.; RouzaudSández, O.; Plascencia-Jatomea, M.; Ezquerra-Brauer, J.M. Giant squid skin gelatin: Chemical composition and biophysical characterization. Food Res. Int. 2011, 44, 3243–3249. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Ghosh, G.; Barman, R.; Mukherjee, A.; Ghosh, U.; Ghosh, S.; Fernandez, G. Control over Multiple Nano-and Secondary Structures in Peptide Self-Assembly. Angew. Chem. 2022, 1, e202113403. [Google Scholar]
- Hanna, S.; Katarzyna, S.; Julia, W.; Anna, W.-P.; Ilona, K. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar]
- Jackson, M.; Choo, L.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim. Biophys. Acta 1995, 1270, 1–6. [Google Scholar] [CrossRef]
- Sha, X.M.; Hu, Z.Z.; Tu, Z.C.; Zhang, L.Z.; Xiao, H. The identification of three mammalian gelatins by liquid chromatography-high resolution mass spectrometry. LWT Food Sci. Technol. 2018, 89, 74–86. [Google Scholar] [CrossRef]
- Eenschooten, C.; Guillaumie, F.; Kontogeorgis, G.M.; Stenby, E.H.; SchwachAbdellaoui, K. Preparation and structural characterisation of novel and versatile amphiphilic octenyl succinic anhydride–modified hyaluronic acid derivatives. Carbohydr. Polym. 2010, 79, 597–605. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, L.; Wang, Y.; Liang, Y.; Zhong, G. Preparation and properties of konjac glucomannan octenyl succinate modified by microwave method. Food Hydrocoll. 2014, 38, 205–210. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C.; Herrera, A.; Prakash, O. Study of octenyl succinic anhydride modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011, 83, 407–413. [Google Scholar] [CrossRef]
- Hu, F.Q.; Ren, G.F.; Yuan, H.; Du, Y.Z.; Zeng, S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf. Biointerfaces 2006, 50, 97–103. [Google Scholar] [CrossRef]
- Li, J.; Geng, S.; Liu, B.; Wang, H.; Liang, G. Self-assembled mechanism of hydrophobic amino acids and β-cyclodextrin based on experimental and computational methods. Food Res. Int. 2018, 112, 136–142. [Google Scholar] [CrossRef]
- Mohammadian, M.; Moghadam, M.; Salami, M.; Emam-Djomeh, Z.; Alavi, F.; Momen, S.; Moosavi-Movahedi, A.A. Whey protein aggregates formed by non-toxic chemical cross-linking as novel carriers for curcumin delivery: Fabrication and characterization. J. Drug Deliv. Sci. Technol. 2020, 56, 101531. [Google Scholar] [CrossRef]
- Ketnawa, S.; Martínez-Alvarez, O.; Benjakul, S.; Rawdkuen, S. Gelatin hydrolysates from farmed Giant catfish skin using alkaline proteases and its antioxidative function of simulated gastro-intestinal digestion. Food Chem. 2016, 192, 34–42. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakula, S.; Kishimura, H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem. 2011, 46, 318–327. [Google Scholar] [CrossRef]
- Carmelo-Luna, F.J.; Mendoza-Wilson, A.M.; Montfort, G.R.C.; Lizardi-Mendoza, J.; Madera-Santana, T.; Lardizábal-Gutiérrez, D.; Quintana-Owen, P. Synthesis and experimental/computational characterization of sorghum procyanidins-gelatin nanoparticles. Bioorganic Med. Chem. 2021, 42, 116240. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, S.; Zhang, Y.; Zheng, Y.; Shi, W.; Wang, X.; Zhong, J. Effects of Antioxidant Types on the Stabilization and In Vitro Digestion Behaviors of Silver Carp Scale Gelatin-Stabilized Fish Oil-Loaded Emulsions. Colloids Surf. B Biointerfaces 2022, 217, 112624. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Regenstein, J.M. Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. J. Food Sci. 2005, 70, c392–c396. [Google Scholar] [CrossRef]
- Chen, F.P.; Ou, S.Y.; Tang, C.H. Core-shell soy protein-soy polysaccharide complex (nano) particles as carriers for improved stability and sustained release of curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, L.S.; Tan, D.C.W.; Moochhala, S.M.; Yang, Y.Y. Mathematical modeling and in vitro study of controlled drug release via a highly swellable and dissoluble polymer matrix: Polyethylene oxide with high molecular weights. J. Control. Release 2005, 102, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Zhang, J.; Zheng, Z.; Kaplan, D.; Li, G.; Wang, X. Oral delivery of curcumin using silk nano-and microparticles. ACS Biomater. Sci. Eng. 2018, 4, 3885–3894. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, J.; Rao, Z.; Hu, J.; Wang, Q.; Sun, Y.; Lei, X.; Zhao, J.; Zeng, K.; Xu, Z.; et al. Study on the stability and oral bioavailability of curcumin loaded (-)-epigallocatechin-3-gallate/poly(N-vinylpyrrolidone) nanoparticles based on hydrogen bonding-driven self-assembly. Food Chem. 2022, 378, 132091. [Google Scholar] [CrossRef]





| Sample | Secondary Structure (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| FSG | 13 ± 2 a | 52 ± 3 a | 20 ± 1 a | 15 ± 1 a |
| OFSG1 | 27 ± 2 b | 35 ± 3 b | 22 ± 3 ab | 16 ± 2 a |
| OFSG2 | 28 ± 1 b | 31 ± 4 b | 26 ± 2 b | 15 ± 1 a |
| Sample | Particle Size (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| FSG | 1408.3 ± 84.0 a | 0.824 ± 0.070 a | −25.2 ± 2.27 c |
| OFSG1 | 1270.7 ± 79.5 b | 0.659 ± 0.063 b | −11.4 ± 0.73 a |
| OFSG2 | 350.0 ± 31.7 d | 0.584 ± 0.048 b | −12.2 ± 1.31 a |
| CL-FSG | 575.5 ± 65.4 c | 0.427 ± 0.033 c | −18.3 ± 0.97 b |
| CL-OFSG1 | 214.5 ± 32.3 e | 0.347 ± 0.012 c | −20.3 ± 2.02 b |
| CL-OFSG2 | 139.5 ± 30.6 e | 0.248 ± 0.022 d | −21.0 ± 2.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Li, H.; Wan, A.W.M.; Ren, T.; Lei, Z.; Liu, J. Curcumin-Loaded Self-Assembly Constructed by Octenylsuccinate Fish (Cyprinus carpio L.) Scale Gelatin: Preparation and Characterization. Foods 2022, 11, 2911. https://doi.org/10.3390/foods11182911
Yu X, Li H, Wan AWM, Ren T, Lei Z, Liu J. Curcumin-Loaded Self-Assembly Constructed by Octenylsuccinate Fish (Cyprinus carpio L.) Scale Gelatin: Preparation and Characterization. Foods. 2022; 11(18):2911. https://doi.org/10.3390/foods11182911
Chicago/Turabian StyleYu, Xiaoyan, Haoxin Li, Aida Wan Mustapha Wan, Tingyuan Ren, Zunguo Lei, and Jia Liu. 2022. "Curcumin-Loaded Self-Assembly Constructed by Octenylsuccinate Fish (Cyprinus carpio L.) Scale Gelatin: Preparation and Characterization" Foods 11, no. 18: 2911. https://doi.org/10.3390/foods11182911
APA StyleYu, X., Li, H., Wan, A. W. M., Ren, T., Lei, Z., & Liu, J. (2022). Curcumin-Loaded Self-Assembly Constructed by Octenylsuccinate Fish (Cyprinus carpio L.) Scale Gelatin: Preparation and Characterization. Foods, 11(18), 2911. https://doi.org/10.3390/foods11182911