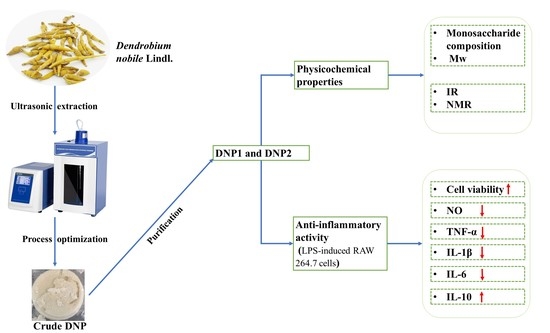
Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Optimal Design of Ultrasonic Extraction Process for DNP
2.2.1. Ultrasonic Extraction Procedure
2.2.2. Single Factor Design
2.2.3. RMS Optimization Design
2.3. Isolation and Purification of DNP
2.4. Structural Characterization of DNPs
2.4.1. Measurement of Monosaccharide Composition and Mw of DNPs
2.4.2. Spectra Analysis of DNPs
2.5. Anti-Inflammatory Activity Assay
2.5.1. Cell Culture
2.5.2. Cell Proliferation and Cytotoxicity Assay
2.5.3. Determination of NO and Cytokine Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Single Factor Experiments Assessment
3.2. RSM Optimization of Ultrasonic Extraction
3.2.1. Fitting the Model
3.2.2. Analysis of Response Surfaces
3.3. The Physicochemical Properties of DNPs
3.4. Structure Analysis of DNPs
3.5. Anti-Inflammatory Activity
3.5.1. Cell Proliferation and Cytotoxicity Assessment
3.5.2. Effect of DNPs on the Content of NO and Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, J.; Jiang, L.S.; Zhang, Y.; Tian, Y.; Li, L.S.; Lu, Y.L.; Yang, W.J.; Shi, J.S. Dendrobium nobile Lindl. Alkaloids decreases the level of intracellular beta-amyloid by improving impaired autolysosomal proteolysis in APP/PS1 Mice. Front. Pharmacol. 2018, 9, 1479. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.Q.; Chen, Y.; Li, W.; Lu, Y.L. Anti-aging properties of Dendrobium nobile Lindl.: From molecular mechanisms to potential treatments. J. Ethnopharmacol. 2020, 257, 112839. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Yu, Q.S.; Wang, G.; Tan, J.W.; Liu, S.; Pu, S.R.; Chen, W.C.; Xie, P.; Zhang, Y.X.; Zhang, J.; et al. Effects of non-thermal plasma treatment on the polysaccharide from Dendrobium nobile Lindl. and its immune activities in vitro. Int. J. Biol. Macromol. 2020, 153, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.X.; Guo, Q.B.; Wang, J.Q.; Cui, S.W. Structural characterization and conformational properties of a polysaccharide isolated from Dendrobium nobile Lindl. Food Hydrocolloid. 2020, 98, 104904. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.Y.; Liu, M.; Chen, F.X.; Yang, W.J.; Chang, X.N.; Liu, N.; Zhao, Y.Y.; Wang, J.; Chen, X.F. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohyd. Res. 2020, 494, 108037. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.B.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.X.; Wang, P.; Ma, C.Y.; He, G.H.; Rahman, M.R.T. Optimization of PEG-based extraction of polysaccharides from Dendrobium nobile Lindl. and bioactivity study. Int. J. Biol. Macromol. 2016, 92, 1057–1066. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.C.; Liu, W.Q.; Qi, Q.; Hu, X.; Li, S.Y.; Lei, J.D.; Rong, L. Extraction of polysaccharide from Dendrobium nobile Lindl. by subcritical water extraction. ACS Omega 2019, 4, 20586–20594. [Google Scholar] [CrossRef]
- Jia, Y.N.; Gao, X.D.; Xue, Z.H.; Wang, Y.J.; Lu, Y.P.; Zhang, M.; Panichayupakaranant, P.; Chen, H.X. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int. J. Biol. Macromol. 2020, 163, 1640–1648. [Google Scholar] [CrossRef]
- Guo, L.A.; Kong, N.; Zhang, X.Y.; Ma, H.L. Multimode ultrasonic extraction of polysaccharides from maca (Lepidium meyenii): Optimization, purification, and in vitro immunoregulatory activity. Ultrason Sonochem. 2022, 88, 106062. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Maan, A.A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-related Matrices. Food Rev. Int. 2022, 38, 1166–1196. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.L.; Zhao, R.Q.; Gao, J.; Kimatu, B.M.; Hu, Q.H.; Chen, G.T.; Zhao, L.Y. Effects of ultrasound-assisted extraction on antioxidant activity and bidirectional immunomodulatory activity of Flammulina velutipes polysaccharide. Int. J. Biol. Macromol. 2019, 140, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Salauddin, M.; Mukherjee, A.; Shariati, M.A.; Rebezov, M.; Tretyak, L.; Pateiro, M.; Lorenzo, J.M. Application of bio-inspired optimization algorithms in food processing. Curr. Res. Food Sci. 2022, 5, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohyd. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Li, S.F.; Wang, A.J.; Liu, L.N.; Tian, G.R.; Xu, F.F. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389. [Google Scholar] [CrossRef]
- Wu, D.M.; Chen, S.G.; Ye, X.Q.; Zheng, X.L.; Ahmadi, S.; Hu, W.W.; Yu, C.X.; Cheng, H.; Linhardt, R.J.; Chen, J.L. Enzyme-extracted raspberry pectin exhibits a high-branched structure and enhanced anti-inflammatory properties than hot acid-extracted pectin. Food Chem. 2022, 383, 132387. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhang, Y.; He, L.; Cheng, J.W.; Li, J.H.; Tao, W.Y.; Mao, G.Z.; Zhang, H.; Linhardt, R.J.; Ye, X.Q.; et al. Structural characterization and anti-proliferative activities of partially degraded polysaccharides from peach gum. Carbohyd. Polym. 2019, 203, 193–202. [Google Scholar] [CrossRef]
- Zhu, J.X.; Chen, Z.Y.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.R.; Hu, X.C.; Wei, X.L.; Wang, Y.F. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Huo, J.X.; Zhong, S.; Zhu, J.X.; Li, Y.G.; Li, X.J. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohyd. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Huang, D.; Hou, X.; Zhang, D.W.; Zhang, Q.; Yan, C.Y. Two novel polysaccharides from rhizomes of Cibotium barometz promote bone formation via activating the BMP2/SMAD1 signaling pathway in MC3T3-E1 cells. Carbohyd. Polym. 2020, 231, 115732. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wang, L.; Ettoumi, F.E.; Javed, M.; Li, L.; Lin, X.Y.; Xu, Y.Q.; Lu, Y.T.; Shao, X.F.; Luo, Z.S. Ultrasonic-assisted green extraction of peach gum polysaccharide for blue-emitting carbon dots synthesis. Sustain. Chem. Pharm. 2021, 24, 100555. [Google Scholar] [CrossRef]
- Wang, K.J.; Guo, J.T.; Cheng, J.X.; Zhao, X.H.; Ma, B.H.; Yang, X.B.; Shao, H.J. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Xu, F.R.; Li, J.L.; Li, J.; Mo, C.; Zhao, M.; Wang, L.F. Ultrasonic-assisted extraction of polysaccharides from coix seeds: Optimization, purification, and in vitro digestibility. Food Chem. 2022, 374, 131636. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cui, P.; Ge, S.; Cai, X.; Li, Q.; Xue, H. Ultrasound assisted aqueous two-phase extraction of polysaccharides from Cornus officinalis fruit: Modeling, optimization, purification, and characterization. Ultrason Sonochem. 2022, 84, 105966. [Google Scholar] [CrossRef]
- Zhu, J.X.; Chen, Z.Y.; Chen, L.; Yu, C.; Wang, H.X.; Wei, X.L.; Wang, Y.F. Comparison and structural characterization of polysaccharides from natural and artificial Se-enriched green. Int. J. Biol. Macromol. 2019, 130, 388–398. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zhang, H.; Shi, L.J.; Li, Y.; Tuerhong, M.; Abudukeremu, M.; Cui, J.L.; Li, Y.H.; Jin, D.Q.; Xu, J.; et al. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Carbohyd. Polym. 2021, 273, 118496. [Google Scholar] [CrossRef]
- Yang, N.X.; Wang, D.P.; Geng, Y.Y.; Man, J.M.; Gao, Y.Y.; Hang, Y.; Zheng, H.J.; Zhang, M.S. Structure, physicochemical characterisation and properties of pectic polysaccharide from Premma puberula pamp. Food Hydrocolloid. 2022, 128, 107550. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xin, Y.; Yin, J.Y.; Huang, X.J.; Wang, J.Q.; Hu, J.L.; Geng, F.; Nie, S.P. Revealing the architecture and solution properties of polysaccharide fractions from Macrolepiota albuminosa (Berk.) Pegler. Food Chem. 2022, 368, 130772. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Song, X.Y.; Jiang, Y.Z.; Yao, J.R.; Jiang, Y.; Li, Z.; Dai, F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris Radix et Rhizoma. Carbohyd. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.Z.; Zhou, C.C.; Xu, T.L.; Chen, X.Y.; Wu, Q.Z.; Zhang, K.F.; Li, Y.; Li, D.X.; Chen, Y. Effects of ultra-high pressure treatment on structure and bioactivity of polysaccharides from large leaf yellow tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef]
- Li, F.; Cui, S.H.; Zha, X.Q.; Bansal, V.; Jiang, Y.L.; Asghar, M.N.; Wang, J.H.; Pan, L.H.; Xu, B.F.; Luo, J.P. Structure and bioactivity of a polysaccharide extracted from protocorm-like bodies of Dendrobium huoshanense. Int. J. Biol. Macromol. 2015, 72, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.T.; Li, J.Y.; Yang, X.B.; Yang, L.; Xu, J.Y.; Yan, S.; Lv, Y.F.; Ren, F.C.; Hu, J.M.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohyd. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.H.; Yin, Z.H.; Liu, X.P.; Ma, C.Y.; Wang, J.M.; Zhang, Y.; Kang, W.Y. A glucomannogalactan from Pleurotus geesteranus: Structural characterization, chain conformation and immunological effect. Carbohyd. Polym. 2022, 287, 119346. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural characterization and anti-inflammatory activity of a polysaccharide from the lignified okra. Carbohyd. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, I.D.; Caillot, A.R.C.; Palhares, L.C.G.F.; Santana, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of polysaccharides from Cabernet Franc, Cabernet Sauvignon and Sauvignon Blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Carbohyd. Polym. 2018, 186, 91–99. [Google Scholar] [CrossRef]
- Xi, Y.W.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.M.; Lin, C.; Lei, B. Bioactive anti-inflammatory, antibacterial, antioxidative silicon-based nanofibrous dressing enables cutaneous tumor photothermo-chemo therapy and infection-induced wound healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ding, Y.Y.; Tian, Y.; Yang, R.X.; Quan, M.L.; Tong, Z.Y.; Zhang, X.L.; Luo, D.; Chi, Z.; Liu, C.U. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effects of sulfated chitosans on RAW 264.7 mouse macrophages via the activation of PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2018, 108, 1310–1321. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, S.Y.; Li, Y.L.; Wang, F.; Yang, X.J.; Yao, J.H. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int. J. Biol. Macromol. 2013, 60, 248–252. [Google Scholar] [CrossRef]
- Bai, Y.J.; Jia, X.C.; Huang, F.; Zhang, R.F.; Dong, L.H.; Liu, L.; Zhang, M.W. Structural elucidation, anti-inflammatory activity and intestinal barrier protection of longan pulp polysaccharide LPIIa. Carbohyd. Polym. 2020, 246, 116532. [Google Scholar] [CrossRef] [PubMed]






| Level | Independent Factors | ||
|---|---|---|---|
| X1 (Liquid–Solid Ratio)/mL·g−1 | X2 (Ultrasonic Times)/min | X3 (Ultrasonic Power)/W | |
| −1 | 30 | 20 | 300 |
| 0 | 40 | 30 | 400 |
| 1 | 50 | 40 | 500 |
| Run | Factors | Extraction Yield/% | ||
|---|---|---|---|---|
| X1 (Liquid–Solid Ratio)/(mL·g−1) | X2 (Ultrasonic Time)/min | X3 (Ultrasonic Power)/W | ||
| 1 | 30 | 20 | 400 | 3.49 |
| 2 | 50 | 20 | 400 | 4.63 |
| 3 | 30 | 40 | 400 | 3.62 |
| 4 | 50 | 40 | 400 | 3.22 |
| 5 | 30 | 30 | 300 | 3.16 |
| 6 | 50 | 30 | 300 | 3.33 |
| 7 | 30 | 30 | 500 | 3.00 |
| 8 | 50 | 30 | 500 | 3.63 |
| 9 | 40 | 20 | 300 | 4.06 |
| 10 | 40 | 40 | 300 | 3.91 |
| 11 | 40 | 20 | 500 | 4.41 |
| 12 | 40 | 40 | 500 | 3.48 |
| 13 | 40 | 30 | 400 | 5.07 |
| 14 | 40 | 30 | 400 | 5.05 |
| 15 | 40 | 30 | 400 | 5.24 |
| 16 | 40 | 30 | 400 | 5.54 |
| 17 | 40 | 30 | 400 | 5.58 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.0012 | 9 | 0.0001 | 32.29 | <0.0001 ** |
| X1-Liquid–Solid Ratio | 0.0000 | 1 | 0.0000 | 7.10 | 0.0322 * |
| X2-Ultrasonic Time | 0.0001 | 1 | 0.0001 | 16.80 | 0.0046 ** |
| X3-Ultrasonic Power | 2.720 × 10−8 | 1 | 2.720 × 10−6 | 0.0065 | 0.9378 |
| X1X2 | 0.0001 | 1 | 0.0001 | 14.33 | 0.0068 ** |
| X1X3 | 5.137 × 10−6 | 1 | 5.137 × 10−6 | 1.24 | 0.3029 |
| X2X3 | 0.0000 | 1 | 0.0000 | 3.66 | 0.0973 |
| X12 | 0.0005 | 1 | 0.0005 | 126.06 | <0.0001 ** |
| X22 | 0.0001 | 1 | 0.0001 | 18.62 | 0.0035 ** |
| X32 | 0.0003 | 1 | 0.0003 | 80.48 | <0.0001 ** |
| Residual | 0.0000 | 7 | 4.156 × 10−6 | ||
| Lack of Fit | 1.172 × 10−6 | 3 | 3.906 × 10−7 | 0.056 | 0.9803 |
| Pure Error | 0.0000 | 4 | 6.980 × 10−6 | ||
| Cor Total | 0.0012 | 16 | |||
| R2 | 0.9765 | ||||
| Adj-R2 | 0.9462 | ||||
| C. V. % | 4.92 | ||||
| Std. Dev | 0.002039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Shi, X.; Zhang, L.; Yao, L.; Cen, L.; Li, L.; Lv, Y.; Wei, C. Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity. Foods 2022, 11, 2957. https://doi.org/10.3390/foods11192957
Chen H, Shi X, Zhang L, Yao L, Cen L, Li L, Lv Y, Wei C. Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity. Foods. 2022; 11(19):2957. https://doi.org/10.3390/foods11192957
Chicago/Turabian StyleChen, Hang, Xueqin Shi, Lin Zhang, Li Yao, Lanyan Cen, Lian Li, Yiyi Lv, and Chaoyang Wei. 2022. "Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity" Foods 11, no. 19: 2957. https://doi.org/10.3390/foods11192957
APA StyleChen, H., Shi, X., Zhang, L., Yao, L., Cen, L., Li, L., Lv, Y., & Wei, C. (2022). Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity. Foods, 11(19), 2957. https://doi.org/10.3390/foods11192957