Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
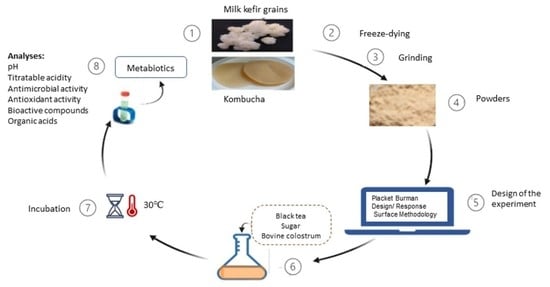
2.1. Milk Kefir Grains and Kombucha Membranes as Starter Cultures
2.2. Co-Fermentation with Starter Cultures Derived from Kombucha Membranes and Milk Kefir Grains
2.3. The Fermented Products’ (FPs) Analysis
2.3.1. pH and Titratable Acidity (TA)
2.3.2. Antifungal Properties of the FPs
2.3.3. Antibacterial Properties of the FPs
2.3.4. Antioxidant Properties of the FPs
2.4. The Design and Optimization of the Fermentation
2.4.1. Plackett–Burman Design of the Experiments
2.4.2. Response Surface Methodology
2.5. Organic Acids and Phenolic Compounds Analysis
2.5.1. Organic Acids Assay
2.5.2. Phenolic Compounds Assessment
2.6. Statistical Analysis
3. Results and Discussions
3.1. Selection of the Most Important Fermentation Parameters by PB Analysis
3.2. Optimization of the Fermentation Process by Response Surface Methodology (RSM)
3.3. The Organic Acids and Phenolic Content of the Fermented Product
3.3.1. Organic Acids’ Content
3.3.2. Polyphenols and Flavonoids Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, R. Studies on bioactive compounds from microorganisms. Int. J. Sci. Eng. Res. 2015, 6, 1225–1233. [Google Scholar]
- Ghosh, J.; Sil, P.C. Natural Bioactive Molecules: Mechanism of Actions and Perspectives in Organ Pathophysiology. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 458–477. [Google Scholar]
- Jawed, K.; Yazdani, S.S.; Koffas, M.A. Advances in the development and application of microbial consortia for metabolic engineering. Metab. Eng. Commun. 2019, 9, e00095. [Google Scholar] [CrossRef] [PubMed]
- Avila-Reyes, S.V.; Márquez-Morales, C.E.; Moreno-León, G.R.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Solorza-Feria, J.; García-Armenta, E.; Villalobos-Espinosa, J.C. Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Appl. Sci. 2022, 12, 2459. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Geng, S.; Espinach, F.X.; Oksman, K.; Vilaseca, F. Bacterial cellulose network from kombucha fermentation impregnated with emulsion-polymerized poly(Methyl methacrylate) to form nanocomposite. Polymers 2021, 13, 664. [Google Scholar] [CrossRef]
- Ciucan, T.; Oancea, A.; Matei, F. Health benefits of fermented colostrum—A review. Sci. Bull. Ser. F. Biotechnol. 2021, XXV, 99–108. [Google Scholar]
- Gao, X.; Li, B. Chemical and microbiological characteristics of kefir grains and their fermented dairy products: A review. Cogent Food Agric. 2016, 2, 1272152. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef] [Green Version]
- London, C. Functional Foods that Boost the Immune System. In Functional Food Product Development; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 293–321. [Google Scholar]
- Hyrslova, I.; Krausova, G.; Michlova, T.; Kana, A.; Curda, L. Fermentation ability of bovine colostrum by different probiotic strains. Fermentation 2018, 6, 93. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Mansour, C.; Ben Jeddou, K.; Machreki, Y.; Kouidhi, B.; Abdulhakim, J.A.; Chaieb, K. Aflatoxin B1 degradation by microorganisms isolated from Kombucha culture. Toxicon 2020, 179, 76–83. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha tea—A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Tran, T.; Romanet, R.; Roullier-Gall, C.; Verdier, F.; Martin, A.; Schmitt-Kopplin, P.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Non-Targeted Metabolomic Analysis of the Kombucha Production Process. Metabolites 2022, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Plakunov, V.K. Biofilm-“city of microbes” or an analogue of multicellular organisms? Microbiology 2007, 76, 125–138. [Google Scholar] [CrossRef]
- Cotârleț, M.; Vasile, A.M.; Cantaragiu, A.M.; Gaspar-Pintiliescu, A.; Craciunescu, O.; Oancea, A.; Moraru, A.; Moraru, I.; Bahrim, G.E. Colostrum-derived bioactive peptides obtained by fermentation with kefir grains enriched with selected yeasts. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2019, 43, 54–68. [Google Scholar] [CrossRef]
- Mirani, A.; Goli, M. Optimization of cupcake formulation by replacement of wheat flour with different levels of eggplant fiber using response surface methodology. Food Sci. Technol. 2022, 42, 1–9. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Turturică, M.; Rocha, J.M.; Bahrim, G.E. Statistical approach to potentially enhance the postbiotication of gluten-free sourdough. Appl. Sci. 2021, 11, 5306. [Google Scholar] [CrossRef]
- Cotârleț, M.; Stănciuc, N.; Bahrim, G.E. Yarrowia lipolytica and lactobacillus paracasei solid state fermentation as a valuable biotechnological tool for the pork lard and okara’s biotransformation. Microorganisms 2020, 8, 1098. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Moraes Filho, M.L.; Busanello, M.; Garcia, S. Optimization of the fermentation parameters for the growth of Lactobacillus in soymilk with okara flour. LWT-Food Sci. Technol. 2016, 74, 456–464. [Google Scholar] [CrossRef]
- Vohra, B.M.; Fazry, S.; Sairi, F.; Babul-Airianah, O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of Kombucha. Malays. J. Fundam. Appl. Sci. 2019, 15, 298–302. [Google Scholar] [CrossRef]
- Cawthray, G.R. An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J. Chromatogr. A 2003, 1011, 233–240. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA-J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Khaleil, M.M. A bioprocess development study of polyphenol profile, antioxidant and antimicrobial activities of kombucha enriched with Psidium guajava L. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1204–1210. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha Tea: Metabolites. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 966–977. [Google Scholar]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Lin, Y.Y.; Chen, M.J.; Chen, L.J.; Lin, C.W. Antioxidative activities of kefir. Asian-Australas. J. Anim. Sci. 2005, 18, 567–573. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.R.; Ibrahim, R.A.; Moustafa, A.H.; Ismaiel, A.A.; Abou Zeid, A.; Enan, G. Chemical constitution and antimicrobial activity of kefir fermented beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Kang, I.B.; Chon, J.W.; Song, K.Y.; Seo, K.H. Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean J. Food Sci. Anim. Resour. 2016, 36, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Shiravani, Z.; Aliakbarlu, J.; Tajik, H. Antibacterial activity of acetic and lactic acid against Listeria monocytogenes and their effect on the intracellular constituent release. Feyz J. Kashan Univ. Med. Sci. 2017, 21, 162–169. [Google Scholar]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Alieva, E.V.; Boltacheva, K.M.; Timchenko, L.D.; Bondareva, N.I.; Dobrynya, Y.M. Antibacterial potential and prospects for kombucha use. Ulyanovsk Med. Biol. J. 2018, 4, 166–171. [Google Scholar] [CrossRef]
- César, J.; Júnior, S.; Meireles Mafaldo, I.; De Lima Brito, I.; Tribuzy, A.M.; Cordeiro, M. Kombucha: Formulation, chemical composition, and therapeutic potentialities. Curr. Res. Food Sci. 2022, 5, 360–365. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha tea beverage: Microbiological characteristic, antioxidant activity, and phytochemical composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. de Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 2001, 34, 223–227. [Google Scholar] [CrossRef]


| Independent Variables | Minimum Value, −1 | Maximum Value, +1 |
|---|---|---|
| A, Concentration of black tea, % (w/v) | 1.0 | 3.0 |
| B, Concentration of sugar, % (w/v) | 5.0 | 10 |
| C, Concentration of colostrum, % (w/v) | 1.0 | 5.0 |
| D, Fermentation time, days | 5.0 | 7.0 |
| E, Concentration of the freeze-dried culture derived from SCOBY-based membranes, % (w/v) | 0.2 | 0.3 |
| F, Concentration of the freeze-dried culture derived from milk kefir grains, % (w/v) | 0.2 | 0.3 |
| Independent Variables | Levels of Variation | ||||
|---|---|---|---|---|---|
| −1 | 0 | +1 | −α | +α | |
| A, Concentration of black tea, % (w/v) | 1.0 | 2.0 | 3.0 | 0.367 | 3.633 |
| C, Concentration of colostrum, % (w/v) | 1.0 | 3.0 | 5.0 | −0.266 | 6.266 |
| F, Concentration of milk kefir grain, %(w/v) | 0.1 | 0.2 | 0.3 | 0.0367 | 0.3633 |
| Sample | Independent Variables * | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | pH | Titratable Acidity, °Th | Antioxidant Activity, μM TE/mL | Antibacterial Activity against B. subtilis, mm | Antifungal Activity against A. niger, % RGI % | |
| 1 | 2.0 | 7.5 | 3.0 | 5.5 | 0.2 | 0.2 | 3.75 | 137.50 | 2.37 | 13.71 | 86.23 |
| 2 | 3.0 | 10.0 | 1.0 | 7.0 | 0.3 | 0.1 | 3.52 | 100.00 | 2.41 | 11.37 | 83.72 |
| 3 | 3.0 | 5.0 | 5.0 | 4.0 | 0.1 | 0.1 | 3.84 | 137.50 | 2.40 | 13.20 | 84.11 |
| 4 | 1.0 | 10.0 | 5.0 | 4.0 | 0.3 | 0.1 | 3.78 | 150.00 | 2.20 | 14.53 | 82.95 |
| 5 | 3.0 | 5.0 | 5.0 | 7.0 | 0.1 | 0.3 | 3.74 | 175.00 | 2.42 | 13.70 | 82.17 |
| 6 | 1.0 | 10.0 | 1.0 | 4.0 | 0.1 | 0.3 | 3.50 | 75.00 | 2.29 | 13.37 | 83.72 |
| 7 | 2.0 | 7.5 | 3.0 | 5.5 | 0.2 | 0.2 | 3.72 | 137.50 | 2.38 | 13.20 | 86.89 |
| 8 | 1.0 | 5.0 | 1.0 | 7.0 | 0.3 | 0.3 | 3.50 | 87.50 | 2.21 | 13.70 | 85.27 |
| 9 | 1.0 | 5.0 | 1.0 | 4.0 | 0.1 | 0.1 | 3.53 | 75.50 | 2.36 | 12.70 | 84.93 |
| 10 | 3.0 | 10.0 | 5.0 | 4.0 | 0.3 | 0.3 | 3.76 | 187.50 | 2.12 | 11.03 | 84.59 |
| 11 | 3.0 | 5.0 | 1.0 | 4.0 | 0.3 | 0.3 | 3.51 | 112.50 | 2.36 | 14.20 | 85.27 |
| 12 | 2.0 | 7.5 | 3.0 | 5.5 | 0.2 | 0.2 | 3.71 | 150.0 | 2.36 | 14.53 | 86.56 |
| 13 | 3.0 | 10.0 | 1.0 | 7.0 | 0.1 | 0.1 | 3.53 | 100.00 | 2.42 | 12.20 | 84.25 |
| 14 | 1.0 | 5.0 | 5.0 | 7.0 | 0.3 | 0.1 | 3.74 | 125.00 | 2.08 | 10.03 | 85.96 |
| 15 | 1.0 | 10.0 | 5.0 | 7.0 | 0.1 | 0.3 | 3.69 | 162.50 | 2.06 | 13.70 | 86.31 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 7 | 16285.7 | 2326.5 | 17.86 | 0.001 |
| Linear | 6 | 15536.6 | 2589.4 | 19.87 | 0.000 |
| Back tea concentration, % | 1 | 1564.1 | 1564.1 | 12.00 | 0.010 |
| Sugar concentration, % | 1 | 320.3 | 320.3 | 2.46 | 0.161 |
| Colostrum concentration, % | 1 | 12480.7 | 12480.7 | 95.79 | 0.000 |
| Time of fermentation, days | 1 | 12.0 | 12.0 | 0.09 | 0.770 |
| Kombucha starter culture concentration, % | 1 | 114.1 | 114.1 | 0.88 | 0.381 |
| Milk kefir grains starter culture concentration, % | 1 | 1045.3 | 1045.3 | 8.02 | 0.025 |
| Curvature | 1 | 749.1 | 749.1 | 5.75 | 0.048 |
| Error | 7 | 912.1 | 130.3 | ||
| Lack-of-Fit | 5 | 807.9 | 161.6 | 3.10 | 0.262 |
| Run | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A * | C | F | pH | Total activity, °Th | Antioxidant Activity, μM TE/mL | Antibacterial Activity against E.coli, mm | Antibacterial Activity against S.aureus, mm | Antibacterial Activity against B.subtilis, mm | Antifungal Activity against A.niger, % | |
| 1 | 1.00 | 1.00 | 0.30 | 3.55 | 225.00 | 1.30 | 2.17 | 0.00 | 10.50 | 93.73 |
| 2 | 2.00 | 3.00 | 0.20 | 3.71 | 229.37 | 2.01 | 4.00 | 0.14 | 10.05 | 92.95 |
| 3 | 2.00 | 3.00 | 0.20 | 3.80 | 220.00 | 2.12 | 1.83 | 0.00 | 8.55 | 93.15 |
| 4 | 3.00 | 1.00 | 0.30 | 3.71 | 100.00 | 2.47 | 0.00 | 0.00 | 4.83 | 100.00 |
| 5 | 1.00 | 5.00 | 0.30 | 3.49 | 344.80 | 1.94 | 6.50 | 3.83 | 18.50 | 100.00 |
| 6 | 1.00 | 5.00 | 0.10 | 3.79 | 212.62 | 1.31 | 2.67 | 3.52 | 14.50 | 93.38 |
| 7 | 3.00 | 5.00 | 0.30 | 3.76 | 250.00 | 2.44 | 3.50 | 0.00 | 10.33 | 100.00 |
| 8 | 1.00 | 1.00 | 0.10 | 3.38 | 229.00 | 1.35 | 2.50 | 0.00 | 11.75 | 90.76 |
| 9 | 2.00 | 3.00 | 0.20 | 3.72 | 234.20 | 2.01 | 4.00 | 0.00 | 11.12 | 92.98 |
| 10 | 3.00 | 5.00 | 0.10 | 3.86 | 187.50 | 2.35 | 0.00 | 0.00 | 8.17 | 90.41 |
| 11 | 2.00 | 3.00 | 0.20 | 3.82 | 215.60 | 2.07 | 2.66 | 0.00 | 7.95 | 94.23 |
| 12 | 3.00 | 1.00 | 0.10 | 3.65 | 193.75 | 2.72 | 2.00 | 0.00 | 7.83 | 100.00 |
| 13 | 2.00 | 3.00 | 0.20 | 3.76 | 243.75 | 2.14 | 2.50 | 0.00 | 9.55 | 93.68 |
| 14 | 3.63 | 3.00 | 0.20 | 3.80 | 150.00 | 2.41 | 0.00 | 0.00 | 6.67 | 100.00 |
| 15 | 2.00 | 6.27 | 0.20 | 3.71 | 308.20 | 2.08 | 5.50 | 3.20 | 16.00 | 100.00 |
| 16 | 2.00 | −0.27 | 0.20 | 3.41 | 225.00 | 2.23 | 2.00 | 0.00 | 8.91 | 100.00 |
| 17 | 2.00 | 3.00 | 0.20 | 3.83 | 206.25 | 2.16 | 3.35 | 0.00 | 9.02 | 93.54 |
| 18 | 2.00 | 3.00 | 0.36 | 3.74 | 212.50 | 2.20 | 2.83 | 0.00 | 7.67 | 100.00 |
| 19 | 2.00 | 3.00 | 0.03 | 3.89 | 162.50 | 2.18 | 1.83 | 0.00 | 7.17 | 91.40 |
| 20 | 0.37 | 3.00 | 0.20 | 3.47 | 238.80 | 0.91 | 5.18 | 3.44 | 16.23 | 96.03 |
| Response | Predicted Value | Experimental Value |
|---|---|---|
| Titratable acidity, °Th | 434.50 | 456.25 ± 0.16 |
| Antioxidant activity, μM TE/ mL | 2.45 | 2.42 ± 0.01 |
| Antibacterial activity against E. coli, mm | 9.50 | 8.67 ± 0.10 |
| Antibacterial activity against S. aureus, mm | 5.07 | 8.00 ± 0.22 |
| Antibacterial activity against B. subtilis, mm | 21.98 | 21.67 ± 0.10 |
| Composite desirability | 0.95 |
| Organic Acids | Concentration, mg/mL | |
|---|---|---|
| Control Sample | Fermented Product | |
| Lactic acid | 7.77 ± 2.14 b | 24.39 ± 0.04 a |
| Citric acid | ND * | 5.77 ± 0.01 a |
| Acetic acid | 9.14 ± 0.00 b | 25.21 ± 0.10 a |
| Butyric acid | 81.63 ± 0.07 a | 67.33 ± 0.05 b |
| Isovaleric acid | 4.36 ± 0.01 a | 4.36 ± 0.01 a |
| Bioactive Compounds | Control Sample, µg/mL | Optimized Sample, µg/mL | ||
|---|---|---|---|---|
| 280 nm | 320 nm | 280 nm | 320 nm | |
| Gallic acid | 170.51 ± 5.73 a | 27.71 ± 0.08 A | 71.40 ± 4.82 b | 7.52 ± 2.34 B |
| Epicatechin | ND | ND | 1062.69 ± 53.50 a | 347.84 ± 50.81 A |
| Caffeic acid | 464.45 ± 49.00 a | 97.16 ± 1.44 A | 314.86 ± 28.10 b | 7.79 ± 2.14 B |
| Chlorogenic acid | 54.66 ± 8.11 a | ND | ND | ND |
| p-Coumaric acid | 28.89 ± 0.11 a | ND | ND | ND |
| Quercetin | ND | ND | 18.20 ± 0.02 a | ND |
| Apigenin | ND | ND | 0.22 ± 0.00 a | ND |
| Isorhamnetin | 4.60 ± 0.10 a | ND | 2.97 ± 0.03 b | ND |
| Kaempferol | 282.30 ± 28.09 a | 9.78 ± 1.00 A | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pihurov, M.; Păcularu-Burada, B.; Cotârleț, M.; Bahrim, G.E. Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties. Foods 2022, 11, 3107. https://doi.org/10.3390/foods11193107
Pihurov M, Păcularu-Burada B, Cotârleț M, Bahrim GE. Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties. Foods. 2022; 11(19):3107. https://doi.org/10.3390/foods11193107
Chicago/Turabian StylePihurov, Marina, Bogdan Păcularu-Burada, Mihaela Cotârleț, and Gabriela Elena Bahrim. 2022. "Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties" Foods 11, no. 19: 3107. https://doi.org/10.3390/foods11193107
APA StylePihurov, M., Păcularu-Burada, B., Cotârleț, M., & Bahrim, G. E. (2022). Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties. Foods, 11(19), 3107. https://doi.org/10.3390/foods11193107