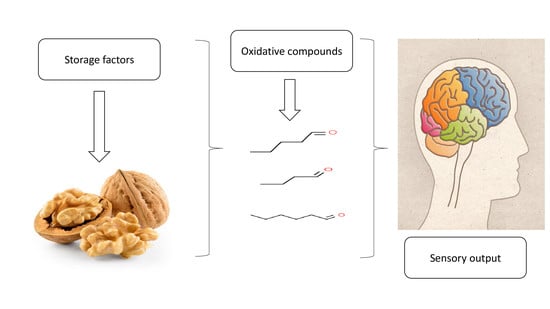
Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Walnut Samples
2.2. Storage Experiment
2.3. Quality Parameters
2.3.1. Kernel Moisture Analysis
2.3.2. Oil Extraction
2.3.3. Evaluation of Peroxide Value, UV Absorbances, Oxidative Stability and Free Fatty Acids
2.4. Profiling of Oil Fatty Acids
2.5. Extraction and Analysis of Oil Tocopherols
2.6. Kernel Total Phenolic Content
2.7. Profiling of Kernel Phenolic Compounds
2.8. Characterization of Kernel and Oil Volatile Compounds
2.9. Sensory Analysis
2.10. Data Analysis
3. Results and Discussion
3.1. Impact of Storage on Quality Parameters
3.1.1. Moisture
3.1.2. Free Fatty Acids and Peroxide Value
3.1.3. UV Absorbances
3.1.4. Oxidative Stability
3.2. Impact of Storage Conditions on Antioxidant Compounds
3.2.1. Changes in Tocopherols
3.2.2. Changes in Kernel Phenolics Profile
3.3. Effect on Walnut Oil Fatty Acid Profile
3.4. Kernel Volatile Changes
3.5. Oil Volatile Changes
3.6. Impact of Storage on Sensory Attributes
3.7. Correlations between Oxidative Volatiles and Sensory Attributes of Kernels and Oils
3.8. Partial Least Squares (PLS) Regression Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.tridge.com/intelligences/walnut/production (accessed on 25 March 2022).
- Abbey, M.; Noakes, M.; Belling, B.G.; Nestel, P.J. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am. J. Clin. Nutr. 1994, 59, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelman, W. Study of the main constituents of some authentic walnut oils. J. Agric. Food Chem. 2005, 53, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Tahmasbian, I.; Zhou, J.; Nevenimo, T.; Hannet, G.; Walton, D.; Randall, B.; Gama, T.; Wallace, H.M. A non-destructive determination of peroxide values, total nitrogen and mineral nutrients in an edible tree nut using hyperspectral imaging. Comput. Electron. Agric. 2018, 151, 492–500. [Google Scholar] [CrossRef]
- Bakkalbaşi, E.; Yılmaz, O.M.; Javidipour, I.; Artik, N. Effects of packaging materials, storage conditions and variety on oxidative stability of shelled walnuts. Food Sci. Technol. 2012, 46, 203–209. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Pandit, R.; Paralikar, P.; Gade, A.; Chaud, M.V.; Alves dos Santos, C. Smart nanopackaging for the enhancement of food shelf life. Environ. Chem. Lett. 2019, 17, 277–290. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality and shelf-life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Tabasum, F.; Umbreen, S.; Syed, Z.H. Nutritional and health benefits of walnuts. J. Pharmacogn. Phytochem. 2018, 7, 1269–1271. [Google Scholar]
- Zwarts, L.; Savage, G.P.; McNeil, D.L. Fatty acid content of New Zealand-grown walnuts (Juglans regia L.). Int. J. Food Sci. Nutr. 1999, 50, 189–194. [Google Scholar] [CrossRef]
- Phatanayindee, S.; Borompichaichartkul, C.; Srzednicki, G.; Craske, J.; Wootto, M. Changes of chemical and physical quality attributes of macadamia nuts during hybrid drying and processing. Dry. Technol. 2012, 30, 1870–1880. [Google Scholar] [CrossRef]
- Grilo, F.S.; Srisaard, Y.; Wang, S.C. Prediction of walnut deterioration using kernel oxidative stability. Foods 2020, 9, 1207. [Google Scholar] [CrossRef]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Grilo, F.S.; Wang, S.C. Walnut (Juglans regia L.) Volatile compounds indicate kernel and oil oxidation. Foods 2021, 10, 329. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventos, R.M.; De la Torre, M.C.; Lopez-Sabater, M.C. The effects of harvest and extraction methods on the antioxidant content (phenolics, alpha-tocopherol, and beta-carotene) in virgin olive oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Salvador, M.D.; Gómez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Int. Food Res. J. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Polari, J.J.; Wang, S.C. Comparative effect of hammer mill screen size and cell wall-degrading enzymes during olive oil extraction. ACS Omega 2020, 5, 6074–6081. [Google Scholar] [CrossRef] [Green Version]
- Ersan, S.; Ustundag, O.G.; Carle, R.; Schweiggert, T. Determination of pistachio (Pistacia veral L.) hull (exo-and mesocarp) phenolics by HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD after ultrasound-assisted extraction. J. Food Compos. Anal. 2017, 62, 103–114. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 16, 2467–2482. [Google Scholar] [CrossRef]
- Shahidi, F.; John, J.A. Oxidative rancidity in nuts. In Improving the Safety and Quality of Nuts; Harris, L.J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 198–229. [Google Scholar] [CrossRef]
- Lin, X.; Wu, J.; Zhu, R.; Chen, P.; Huang, G.; Li, Y.; Ye, N.; Huang, B.; Lai, Y.; Zhang, H.; et al. California almond shelf life: Lipid deterioration during storage. J. Food Sci. 2012, 77, C583–C593. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sedaghat, N. Effect of active edible coating and temperature on quality properties of roasted pistachio nuts during storage. J. Food Process. Preserv. 2019, 43, e14121. [Google Scholar] [CrossRef]
- Buransompob, A.; Tang, J.; Mao, R.; Swanson, B.G. Rancidity of walnuts and almonds affected by short time heat treatments or insect control. J. Food Process. Preserv. 2003, 27, 445–464. [Google Scholar] [CrossRef]
- Sena-Moreno, E.; Pardo, J.E.; Pardo-Giménez, A.; Gómez, R.; Alvarez-Ortí, M. Differences in oils from nuts extracted by means of two pressure systems. Int. J. Food Prop. 2016, 19, 2750–2760. [Google Scholar] [CrossRef] [Green Version]
- Samaniego-Sanchez, C.; Oliveras-Lopez, M.J.; Quesada-Granados, J.J.; Villalon-Mir, M.; Lopez-G Serrana, H. Alterations in picual extra virgin olive oils under different storage conditions. Eur. J. Lipid Sci. Technol. 2012, 114, 194–204. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Orti, M.; Pardo, J.E.; Alvarruiz, A. Storage stability and composition changes of three cold-pressed nut oils under refrigeration and room temperature conditions. Food Chem. 2018, 259, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. J. Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Hussain, N.; Irshad, F.; Jabeen, Z.; Shamsi, I.H.; Li, Z.; Jiang, L. Biosynthesis, structural, and functional attributes of tocopherols in planta; past, present, and future perspectives. J. Agric. Food Chem. 2013, 61, 6137–6149. [Google Scholar] [CrossRef]
- Liu, H.K.; Chen, Y.Y.; Zhang, S.J.; Hu, T.T.; Zhang, Y.H.; Zhao, T.Y.; Kang, Y. The Influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 59–465. [Google Scholar] [CrossRef]
- Korbel, E.; Attal, E.H.; Grabulos, J.; Lluberas, E.; Durand, N.; Morel, G.; Goli, T.; Brat, P. Impact of temperature and water activity on enzymatic and non-enzymatic reactions in reconstituted dried mango model system. Eur. Food Res. Technol. 2013, 237, 39–46. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Yıldız, A.Y.; Karaca, H. Comparison of the oil quality of light and dark walnuts under different storage conditions. J. Oleo Sci. 2021, 70, 615–632. [Google Scholar] [CrossRef]
- Bolling, B.W.; Blumberg, J.B.; Chen, C.Y.O. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [Green Version]
- Zamora, R.; Hidalgo, F.J. The triple defensive barrier of phenolic compounds against the lipid oxidation-induced damage in food products. Trends Food Sci. Technol. 2016, 54, 165–174. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pe, D. Identification of volatile oxidation compounds as potential markers of walnut oil quality. J. Food Sci. 2018, 83, 2745–2752. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Oil composition in stored walnut cultivars-quality and nutritional value. Eur. J. Lipid Sci. Technol. 2015, 117, 338–348. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Biolatto, A.; Rizzo, S.A.; Perez, C.D.; Frusso, E.A.; Carduza, F.; Rossetti, L. Oxidative stability parameters and sensory properties of in-shell “Stuart” pecans (Carya illinoinensis (Wangenh.) K. Koch) stored at different temperatures under non-accelerated conditions. Postharvest Biol. Technol. 2021, 179, 111591. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Erickson, M.C.; Santerre, C.R.; Malingre, M.E. Oxidative stability in raw and roasted pecans: Chemical, physical and sensory measurements. J. Food Sci. 1994, 59, 1234–1238. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F. Tree nuts, composition, phytochemicals and health effects. In Tree nuts; Alasalvar, C., Shahidi, F., Eds.; CRS Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK, 2009; pp. 1–10. [Google Scholar] [CrossRef]
- Available online: https://www.flavornet.org/flavornet.html (accessed on 21 April 2022).
- Mexis, S.F.; Badeka, A.V.; Riganakos, K.A.; Karakostas, K.X.; Kontominas, M.G. Effect of packaging and storage conditions on quality of shelled walnuts. Food Control 2009, 20, 743–751. [Google Scholar] [CrossRef]





| Cultivar | Temp. (C) | Storage Time (Months) | Quality Parameter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture (g/kg) | FFA (g/100 g) | Oil PV (meq O2/kg) | K232 | K268 | Kernel OS (h) | Oil OS (h) | |||
| Howard | |||||||||
| 0 | 4.13 ± 0.3d | 0.01 ± 0.0c | 0.85 ± 0.1c | 0.90 ± 0.0e | 0.09 ± 0.0f | 14.08 ± 0.11a | 2.84 ± 0.07a | ||
| 5 °C | 1 | 4.40 ± 0.2d | 0.02 ± 0.0b | 0.98 ± 0.2bc | 1.25 ± 0.0cb | 0.13 ± 0.0c | 13.10 ± 0.34a | 2.84 ± 0.05a | |
| 2 | 6.87 ± 0.6b | 0.02 ± 0.0b | 1.06 ± 0.2b | 1.28 ± 0.0cb | 0.15 ± 0.0b | 12.07 ± 0.19bc | 2.68 ± 0.03b | ||
| 3 | 7.57 ± 0.3a | 0.03 ± 0.0a | 1.23 ± 0.1b | 1.35 ± 0.0b | 0.15 ± 0.0b | 11.44 ± 0.52c | 2.46 ± 0.01cd | ||
| 4 | 7.88 ± 0.2a | 0.03 ± 0.0a | 1.80 ± 0.0a | 1.55 ± 0.0b | 0.10 ± 0.0e | 10.74 ± 0.37d | 2.51 ± 0.01c | ||
| 23 °C | 1 | 5.4 ± 0.2c | 0.02 ± 0.0b | 0.93 ± 0.3bc | 1.42 ± 0.0d | 0.12 ± 0.0d | 14.07 ± 0.04a | 2.88 ± 0.02a | |
| 2 | 4.4 ± 0.0d | 0.02 ± 0.0b | 1.13 ± 0.1b | 1.56 ± 0.0c | 0.13 ± 0.0cd | 12.59 ± 0.24b | 2.65 ± 0.02b | ||
| 3 | 5.07 ± 0.2c | 0.02 ± 0.0b | 1.06 ± 0.1b | 1.82 ± 0.2b | 0.18 ± 0.0a | 12.22 ± 0.33bc | 2.57 ± 0.02c | ||
| 4 | 5.16 ± 0.6c | 0.03 ± 0.0a | 1.80 ± 0.2a | 2.50 ± 0.1a | 0.18 ± 0.0a | 11.07 ± 0.50cd | 2.48 ± 0.01d | ||
| Chandler | |||||||||
| 0 | 4.22 ± 0.3d | 0.01 ± 0.0c | 0.70 ± 0.2d | 1.06 ± 0.0g | 0.09 ± 0.0b | 14.54 ± 0.50a | 2.97 ± 0.13a | ||
| 5 °C | 1 | 4.33 ± 0.3d | 0.02 ± 0.0b | 1.06 ± 0.1c | 1.24 ± 0.0edf | 0.14 ± 0.0a | 13.97 ± 0.17ba | 2.87 ± 0.02a | |
| 2 | 6.93 ± 0.5a | 0.02 ± 0.0b | 1.06 ± 0.2c | 1.21 ± 0.0e | 0.10 ± 0.0b | 13.09 ± 0.10c | 2.68 ± 0.02b | ||
| 3 | 6.20 ± 0.1b | 0.02 ± 0.0b | 1.33 ± 0.1bc | 1.29 ± 0.0c | 0.10 ± 0.0b | 12.33 ± 0.49d | 2.39 ± 0.05c | ||
| 4 | 7.42 ± 0.1a | 0.03 ± 0.0a | 1.50 ± 0.1ab | 1.39 ± 0.0bc | 0.09 ± 0.0b | 12.07 ± 0.53d | 2.28 ± 0.06d | ||
| 23 °C | 1 | 5.27 ± 0.6bc | 0.01 ± 0.0c | 0.93 ± 0.3bd | 1.27 ± 0.0e | 0.13 ± 0.0a | 14.01 ± 0.42a | 2.72 ± 0.04a | |
| 2 | 4.73 ± 0.6cd | 0.02 ± 0.0cb | 1.13 ± 0.1b | 1.45 ± 0.1d | 0.11 ± 0.0b | 13.06 ± 0.05b | 2.66 ± 0.05a | ||
| 3 | 5.26 ± 0.3bc | 0.02 ± 0.0b | 1.06 ± 0.1b | 1.68 ± 0.1b | 0.11 ± 0.0b | 13.07 ± 0.13b | 2.51 ± 0.03b | ||
| 4 | 4.37 ± 0.3d | 0.03 ± 0.0a | 1.80 ± 0.2a | 1.94 ± 0.0a | 0.13 ± 0.0a | 12.24 ± 0.24c | 2.03 ± 0.03c | ||
| Cultivar | Phenolic Compound (mg/kg) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Howard | Month 0 | Month 1 | Month 2 | Month 3 | Month 4 | Month 1 | Month 2 | Month 3 | Month 4 | |
| Total phenols | 11,119.13 ± 585.3a | 11,508.64 ± 342.8a | 10,852.22 ± 131.5bc | 9645.08 ± 114.0bc | 9094.22 ± 185.7bc | 12,092.99 ± 131.9a | 10,386.31 ± 102.2bc | 9997.83 ± 105.9bc | 10,102.79 ± 519.3c | |
| Gallic acid | 467.47 ± 24.9e | 363.11 ± 0.9d | 616.18 ± 1.2b | 516.31 ± 0.6c | 513.8 ± 32.3ce | 651.47 ± 0.5b | 568.10 ± 1.2c | 549.49 ± 1.7c | 703.03 ± 17.1a | |
| Gallocatechin | 20.87 ± 7.8g | 29.66 ± 0.9f | 77.50 ± 0.9a | 61.28 ± 0.6b | 41.84 ± 0.9d | 76.18 ± 0.4a | 57.50 ± 1.0c | 62.03 ± 1.7b | 34.50 ± 2.9e | |
| Protocatechuic acid | 54.74 ± 9.4b | 68.29 ± 1.0a | 40.02 ± 1.2c | 35.45 ± 0.6d | 27.60 ± 0.7e | 40.60 ± 0.5c | 51.94 ± 0.8b | 29.52 ± 1.8e | 20.74 ± 0.5f | |
| Catechin | 13.91 ± 2.9e | 37.30 ± 1.4b | 23.39 ± 0.9c | 21.50 ± 0.6d | 18.25 ± 1.2e | 25.40 ± 0.9c | 51.46 ± 0.8a | 25.06 ± 1.7c | 35.68 ± 1.9b | |
| Chlorogenic acid | 43.59 ± 3.9b | 50.66 ± 1.0a | 33.06 ± 1.2c | 20.98 ± 0.6e | 42.36 ± 16.6ab | 31.69 ± 0.7c | 27.06 ± 0.9d | 23.82 ± 1.5d | 54.38 ± 1.1a | |
| Vanillic acid | 56.35 ± 1.9f | 197.05 ± 0.8a | 86.22 ± 1.2b | 57.94 ± 0.6f | 60.02 ± 1.7e | 87.58 ± 0.6b | 75.34 ± 1.3d | 62.70 ± 1.8e | 81.53 ± 1.1c | |
| Caffeic acid | 43.40 ± 8.9i | 125.03 ± 0.9h | 222.16 ± 1.2b | 182.79 ± 0.6f | 207.58 ± 7.2d | 211.61 ± 0.6c | 190.92 ± 1.1e | 166.35 ± 1.6g | 240.62 ± 9.1a | |
| Epicatechin | 31.72 ± 6.2e | 67.41 ± 0.7a | 50.51 ± 0.8bc | 44.41 ± 0.6d | 40.77 ± 6.6d | 51.73 ± 0.8bc | 48.14 ± 0.8c | 53.90 ± 1.8b | 32.84 ± 2.9e | |
| Gallocatechin gallate | 10.78 ± 0.2h | 32.29 ± 0.9g | 79.44 ± 1.5e | 62.60 ± 0.6f | 185.99 ± 42.1a | 81.84 ± 0.5de | 86.79 ± 0.8d | 129.42 ± 2.1c | 173.06 ± 0.8b | |
| p-coumaric acid | 134.85 ± 0.4c | 137.76 ± 1.2c | 161.18 ± 1.2a | 124.17 ± 0.6d | 49.07 ± 44.7f | 148.77 ± 0.6b | 121.11 ± 1.0d | 106.21 ± 2.0e | 31.05 ± 2.7g | |
| Epicatechin gallate | 489.97 ± 78.9b | 667.30 ± 0.9a | 194.54 ± 0.9ce | 182.15 ± 0.6d | 164.17 ± 25.9e | 187.43 ± 0.5de | 181.38 ± 1.1d | 189.16 ± 2.1de | 86.15 ± 11.8f | |
| Rutin | 139.29 ± 18.9h | 226.92 ± 1.1g | 671.04 ± 1.2b | 559.58 ± 0.6e | 53.03 ± 0.6i | 680.72 ± 0.5a | 635.77 ± 1.2c | 604.13 ± 1.5d | 256.56 ± 4.7f | |
| Quercetin | 203.16 ± 1.7b | 165.57 ± 0.9e | 194.69 ± 1.1c | 132.64 ± 0.6f | 160.53 ± 9.8e | 191.57 ± 0.2c | 183.19 ± 0.8d | 196.28 ± 1.9bc | 247.73 ± 2.5a | |
| Ellagic acid | 45.98 ± 4.3f | 49.20 ± 1.2f | 96.11 ± 1.2c | 69.74 ± 0.6e | 141.42 ± 7.4a | 85.61 ± 0.9d | 123.69 ± 1.3b | 88.62 ± 2.1c | 154.86 ± 2.8a | |
| Chandler | ||||||||||
| Total Phenol | 12,423.75 ± 401.4a | 11,757.97 ± 224.6a | 11,553.24 ± 293.1a | 9855.02 ± 91.3b | 8939.90 ± 202.6d | 11,449.12 ± 298.9a | 9464.39 ± 147.1c | 8988.20 ± 95.5d | 9077.50 ± 365.8d | |
| Gallic acid | 243.37 ± 20.4h | 506.34 ± 0.8f | 775.09 ± 1.4b | 599.15 ± 0.6d | 639.52 ± 19.7c | 382.27 ± 0.9g | 612.84 ± 1.3c | 545.02 ± 1.3e | 981.68 ± 84.3a | |
| Gallocatechin | 27.01 ± 2.5f | 15.04 ± 0.8g | 93.99 ± 1.9a | 51.44 ± 0.6c | 27.89 ± 2.4e | 21.45 ± 1.1f | 72.52 ± 1.1b | 47.31 ± 1.3d | 31.44 ± 0.6e | |
| Protocatechuic acid | 73.57 ± 5.0a | 76.96 ± 0.9a | 35.26 ± 1.1b | 25.89 ± 0.6c | 27.89 ± 2.4c | 80.38 ± 1.1a | 35.25 ± 1.1b | 26.76 ± 1.3c | 17.59 ± 1.2d | |
| Catechin | 40.01 ± 3.2c | 34.15 ± 1.1d | 54.11 ± 1.3b | 57.02 ± 0.6b | 35.45 ± 4.1d | 20.18 ± 1.1e | 63.89 ± 0.9a | 20.91 ± 1.2e | 52.86 ± 18.4b | |
| Chlorogenic acid | 439.40 ± 39.4a | 42.68 ± 0.8b | 24.22 ± 1.7de | 22.60 ± 1.2de | 17.34 ± 0.8e | 32.60 ± 1.2c | 27.52 ± 1.2dc | 24.07 ± 1.0dec | 20.26 ± 0.8e | |
| Vanillic acid | 68.02 ± 3.3ef | 89.42 ± 0.7bc | 94.95 ± 1.2ab | 85.51 ± 0.6cd | 74.25 ± 2.3e | 73.40 ± 1.1e | 80.28 ± 0.7e | 60.75 ± 0.9f | 121.88 ± 11.5a | |
| Caffeic acid | 250.36 ± 59.9cd | 234.49 ± 0.8e | 316.65 ± 1.4bc | 250.67 ± 0.6de | 279.03 ± 3.5d | 124.90 ± 1.1f | 325.44 ± 1.0bc | 228.99 ± 1.4e | 498.03 ± 32.9a | |
| Epicatechin | 57.67 ± 18.4c | 62.19 ± 0.6a | 39.77 ± 1.7bc | 48.32 ± 0.6b | 33.53 ± 2.7c | 35.04 ± 0.9c | 39.40 ± 1.2c | 40.35 ± 1.3c | 33.95 ± 1.1c | |
| Gallocatechin gallate | 34.09 ± 0.3f | 12.32 ± 0.8g | 76.76 ± 1.5c | 60.42 ± 0.6d | 124.43 ± 19.6b | 50.62 ± 1.2e | 49.43 ± 0.9e | 57.58 ± 0.9de | 208.41 ± 9.8a | |
| p-coumaric acid | 190.62 ± 25.8abc | 134.70 ± 0.9bf | 159.01 ± 1.1c | 142.08 ± 0.6e | 166.91 ± 9.5b | 153.77 ± 1.1dc | 132.87 ± 0.8f | 111.47 ± 1.2g | 244.77 ± 17.4a | |
| Epicatechin gallate | 683.94 ± 59.6a | 620.58 ± 0.8a | 187.68 ± 1.8c | 171.13 ± 0.6d | 185.96 ± 1.4c | 413.22 ± 1.2b | 175.80 ± 1.1d | 186.96 ± 1.3c | 183.27 ± 5.6c | |
| Rutin | 513.06 ± 42.3d | 134.81 ± 0.9e | 708.09 ± 1.8b | 541.23 ± 0.6d | 569.99 ± 23.0d | 212.56 ± 1.1f | 630.56 ± 1.2c | 509.11 ± 0.9d | 891.73 ± 56.8a | |
| Quercetin | 203.69 ± 24.9dc | 215.44 ± 0.8d | 175.92 ± 1.6de | 267.91 ± 0.6b | 167.87 ± 6.3e | 300.27 ± 0.9a | 156.42 ± 0.9f | 143.05 ± 0.8f | 259.21 ± 17.0c | |
| Ellagic acid | 53.57 ± 5.7cd | 50.17 ± 0.9d | 59.88 ± 1.5bc | 54.38 ± 0.6d | 47.86 ± 3.4e | 49.87 ± 1.3e | 76.34 ± 1.1a | 45.40 ± 1.5ef | 44.86 ± 0.9f | |
| Cultivar | Fatty Acid (%) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Howard | Month 0 | Month 1 | Month 2 | Month 3 | Month 4 | Month 1 | Month 2 | Month 3 | Month 4 | |
| C16:0 | 2.29 ± 0.2a | 2.88 ± 0.2a | 2.38 ± 0.3a | 2.29 ± 0.0a | 2.28 ± 0.0a | 1.75 ± 0.2a | 2.61 ± 0.3a | 1.66 ± 0.1a | 1.69 ± 0.0a | |
| C16:1 | 6.43 ± 0.0a | 6.24 ± 0.0a | 6.28 ± 0.0a | 6.33 ± 0.0a | 6.32 ± 0.0a | 6.29 ± 0.0a | 6.29 ± 0.0a | 6.37 ± 0.0a | 6.37 ± 0.0a | |
| C17:0 | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | |
| C17:1 | 2.32 ± 0.0a | 2.20 ± 0.0a | 2.39 ± 0.1a | 2.34 ± 0.0a | 2.34 ± 0.0a | 2.29 ± 0.1a | 2.20 ± 0.1a | 2.35 ± 0.3a | 2.34 ± 0.0a | |
| C18:0 | 3.69 ± 0.1a | 4.72 ± 0.3a | 4.43 ± 0.9a | 4.11 ± 0.0a | 4.10 ± 0.0a | 4.11 ± 0.2a | 4.33 ± 0.1a | 3.62 ± 0.3a | 3.64 ± 0.3a | |
| C18:1 | 9.20 ± 0.2a | 8.42 ± 0.3a | 8.57 ± 0.9a | 8.92 ± 0.2a | 8.93 ± 0.2a | 8.99 ± 0.3a | 8.56 ± 0.3a | 9.49 ± 0.3a | 9.33 ± 0.1a | |
| C18:2 | 59.83 ± 0.1a | 59.61 ± 0.2a | 59.88 ± 0.3a | 60.08 ± 0.1a | 60.11 ± 0.1a | 60.46 ± 0.1a | 59.99 ± 0.1a | 60.58 ± 0.0a | 60.69 ± 0.1a | |
| C18:3 | 15.87 ± 0.0a | 15.56 ± 0.0a | 15.71 ± 0.1a | 15.56 ± 0.0a | 15.56 ± 0.0a | 15.74 ± 0.0a | 15.63 ± 0.0a | 15.54 ± 0.0a | 15.56 ± 0.1a | |
| C22:0 | 0.09 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.5a | 0.08 ± 0.0a | 0.08 ± 0.0a | 0.08 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | |
| C22:1 | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.23 ± 0.0a | 0.22 ± 0.0a | |
| SFA | 6.13 ± 0.3a | 7.75 ± 0.5a | 6.95 ± 1.2a | 6.54 ± 0.0a | 6.51 ± 0.0a | 5.99 ± 0.4a | 7.08 ± 0.4a | 5.43 ± 0.2a | 5.48 ± 0.2a | |
| UFA | 93.87 ± 0.3a | 92.25 ± 0.5a | 93.05 ± 1.2a | 93.46 ± 0.0a | 93.49 ± 0.0a | 94.01 ± 0.4a | 92.92 ± 0.4a | 94.57 ± 0.2a | 94.52 ± 0.2a | |
| MUFA | 18.18 ± 0.2a | 17.08 ± 0.3a | 17.46 ± 0.9a | 17.82 ± 0.2a | 17.81 ± 0.2a | 17.81 ± 0.2a | 17.29 ± 0.2a | 18.45 ± 0.3a | 18.27 ± 0.1a | |
| PUFA | 75.70 ± 0.1a | 75.17 ± 0.2a | 75.59 ± 0.4a | 75.64 ± 0.1a | 75.67 ± 0.1a | 76.19 ± 0.2a | 75.63 ± 0.2a | 76.12 ± 0.0a | 76.25 ± 0.2a | |
| Chandler | ||||||||||
| C16:0 | 2.18 ± 0.5a | 1.59 ± 0.5a | 1.80 ± 0.0a | 2.48 ± 0.2a | 2.45 ± 0.1a | 2.35 ± 0.1a | 1.97 ± 0.0a | 2.42 ± 0.2a | 2.42 ± 0.2a | |
| C16:1 | 5.89 ± 0.0a | 5.93 ± 0.0a | 6.00 ± 0.0a | 5.98 ± 0.0a | 6.03 ± 0.1a | 6.02 ± 0.0a | 5.84 ± 0.2a | 6.07 ± 0.0a | 6.07 ± 0.0a | |
| C17:0 | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | 0.04 ± 0.0a | 0.06 ± 0.0a | 0.06 ± 0.0a | |
| C17:1 | 1.32 ± 1.7a | 2.42 ± 0.1a | 2.18 ± 0.1a | 2.42 ± 0.1a | 2.32 ± 0.0a | 2.41 ± 0.1a | 2.46 ± 0.0a | 2.33 ± 0.0a | 2.33 ± 0.0a | |
| C18:0 | 3.26 ± 1.3a | 4.53 ± 0.5a | 4.42 ± 0.0a | 3.96 ± 0.2a | 4.28 ± 0.3a | 3.86 ± 0.1a | 4.05 ± 0.2a | 4.24 ± 0.3a | 4.24 ± 0.3a | |
| C18:1 | 11.77 ± 2.9a | 9.63 ± 0.5a | 9.58 ± 0.1a | 9.97 ± 0.1a | 9.58 ± 0.4a | 9.77 ± 0.1a | 9.88 ± 0.2a | 9.52 ± 0.4a | 9.52 ± 0.4a | |
| C18:2 | 60.38 ± 0.3a | 60.65 ± 0.2a | 60.81 ± 0.1a | 60.29 ± 0.2a | 60.35 ± 0.1a | 60.25 ± 0.0a | 60.74 ± 0.1a | 60.36 ± 0.1a | 60.36 ± 0.1a | |
| C18:3 | 14.84 ± 0.1a | 14.88 ± 0.1a | 14.82 ± 0.0a | 14.52 ± 0.1a | 14.61 ± 0.1a | 14.97 ± 0.0a | 14.69 ± 0.0a | 14.69 ± 0.0a | 14.69 ± 0.0a | |
| C22:0 | 0.08 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | 0.08 ± 0.0a | 0.08 ± 0.0a | 0.09 ± 0.0a | 0.09 ± 0.0a | |
| C22:1 | 0.23 ± 0.0a | 0.24 ± 0.0a | 0.24 ± 0.0a | 0.24 ± 0.0a | 0.24 ± 0.0a | 0.23 ± 0.0a | 0.24 ± 0.0a | 0.24 ± 0.0a | 0.24 ± 0.0a | |
| SFA | 5.57 ± 0.8a | 6.26 ± 0.1a | 6.37 ± 0.0a | 6.59 ± 0.0a | 6.87 ± 0.4a | 6.35 ± 0.0a | 6.14 ± 0.2a | 6.80 ± 0.5a | 6.80 ± 0.5a | |
| UFA | 94.43 ± 0.8a | 93.74 ± 0.1a | 93.63 ± 0.0a | 93.41 ± 0.0a | 93.13 ± 0.4a | 93.65 ± 0.0a | 93.86 ± 0.2a | 93.20 ± 0.5a | 93.20 ± 0.5a | |
| MUFA | 19.20 ± 1.2a | 18.21 ± 0.3a | 17.99 ± 0.1a | 18.60 ± 0.2a | 18.18 ± 0.1a | 18.43 ± 0.0a | 18.42 ± 0.1a | 18.16 ± 0.4a | 18.16 ± 0.4a | |
| PUFA | 75.23 ± 0.4a | 75.53 ± 0.3a | 75.63 ± 0.1a | 74.81 ± 0.2a | 74.95 ± 0.1a | 75.22 ± 0.0a | 75.44 ± 0.1a | 75.04 ± 0.1a | 75.04 ± 0.1a | |
| Volatile Compound (mg/kg) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Propanol | 2.17 ± 0.6h | 8.42 ± 0.1g | 6.99 ± 0.4f | 18.16 ± 1.9e | 31.99 ± 6.3d | 13.87 ± 1.7e | 59.00 ± 7.1c | 90.82 ± 12.7b | 169.77 ± 30.6a |
| Butanal | 28.55 ± 1.9a | 2.44 ± 0.5fe | 2.39 ± 0.3fe | 3.42 ± 0.1e | 4.36 ± 0.7de | 1.42 ± 0.3g | 5.08 ± 0.6d | 6.99 ± 0.4c | 9.18 ± 1.4b |
| Pentanal | 1.33 ± 0.1g | 11.86 ± 0.3f | 12.47 ± 1.3f | 35.95 ± 2.2e | 48.02 ± 6.2d | 14.11 ± 1.3f | 64.21 ± 5.3c | 109.22 ± 5.5a | 88.82 ± 13.8b |
| Hexanal | 22.77 ± 2.1f | 205.66 ± 31.9d | 260.37 ± 70.7d | 766.62 ± 33.3b | 1319.45 ± 54.8a | 153.59 ± 18.5e | 585.90 ± 52.7c | 1227.73 ± 39.6a | 1238.37 ± 165.7a |
| E-2-Pentenal | 0.11 ± 0.1g | 0.50 ± 0.1dfg | 0.66 ± 0.0efg | 1.11 ± 0.1ef | 1.70 ± 0.2d | 1.10 ± 0.2ef | 3.70 ± 0.3c | 6.58 ± 0.3b | 10.25 ± 0.9a |
| Heptanal | 0.32 ± 0.1e | 1.65 ± 0.2cd | 2.11 ± 0.1c | 4.98 ± 0.6b | 9.32 ± 1.3a | 1.12 ± 0.1de | 3.56 ± 0.2b | 7.88 ± 0.1a | 7.09 ± 0.9a |
| E-2-Hexenal | 0.21 ± 0.0e | 1.02 ± 0.1d | 1.61 ± 0.1d | 5.16 ± 0.2b | 7.50 ± 0.9a | 1.50 ± 0.1d | 3.65 ± 0.3c | 7.06 ± 0.5a | 4.93 ± 0.6b |
| Octanal | 0.19 ± 0.0c | 0.72 ± 0.1b | 0.62 ± 0.1b | 1.17 ± 0.0b | 1.97 ± 0.2a | 0.57 ± 0.1b | 1.05 ± 0.1b | 2.53 ± 0.0a | 2.45 ± 0.9a |
| E-2-Heptenal | 0.30 ± 0.0f | 0.75 ± 0.0e | 0.77 ± 0.1e | 2.18 ± 0.1d | 13.20 ± 0.6a | 1.65 ± 0.1d | 6.84 ± 0.2b | 13.20 ± 0.6a | 4.38 ± 0.9c |
| Nonanal | 0.52 ± 0.0d | 1.76 ± 0.1d | 2.81 ± 0.2c | 5.74 ± 0.9b | 7.98 ± 0.6a | 0.95 ± 0.1d | 1.03 ± 0.1d | 2.27 ± 0.5c | 2.89 ± 0.2c |
| E-2-Octenal | 0.21 ± 0.0f | 0.53 ± 0.1e | 0.70 ± 0.1e | 2.64 ± 0.2d | 7.63 ± 1.6b | 1.15 ± 0.1e | 4.16 ± 0.2c | 11.52 ± 0.3a | 6.27 ± 0.9b |
| Benzaldehyde | 1.23 ± 0.0d | 1.32 ± 0.1cd | 1.87 ± 0.1a | 1.68 ± 0.1b | 1.43 ± 0.1c | 1.15 ± 0.1d | 1.57 ± 0.0c | 1.80 ± 0.1a | 1.87 ± 0.2a |
| E-E-2,4-Nonadienal | 0.02 ± 0.0e | 0.21 ± 0.0d | 0.26 ± 0.0d | 0.73 ± 0.0cb | 1.59 ± 0.2a | 0.09 ± 0.0e | 0.25 ± 0.0d | 0.81 ± 0.0b | 0.58 ± 0.0c |
| 1-penten-3-ol | 3.34 ± 0.1f | 11.85 ± 0.6e | 10.68 ± 0.4e | 26.43 ± 1.7d | 38.42 ± 2.5c | 25.44 ± 1.1d | 86.13 ± 4.4b | 125.67 ± 11.1a | 127.47 ± 17.4a |
| 1-pentanol | 1.31 ± 0.1f | 18.12 ± 0.9e | 38.74 ± 2.5d | 89.79 ± 3.9b | 115.59 ± 10.8a | 14.32 ± 1.0e | 43.64 ± 2.1d | 89.43 ± 5.4b | 62.54 ± 6.5c |
| Z-2-Penten-1-ol | 0.11 ± 0.0e | 0.41 ± 0.0d | 0.60 ± 0.0d | 1.06 ± 0.1c | 2.51 ± 0.3b | 0.47 ± 0.1d | 1.11 ± 0.0c | 2.27 ± 0.1b | 4.01 ± 0.3a |
| 1-hexanol | 3.21 ± 0.3h | 100.88 ± 4.9d | 306.10 ± 22.7c | 520.38 ± 33.6b | 607.47 ± 66.7a | 8.48 ± 0.3g | 13.50 ± 0.5f | 24.92 ± 1.4e | 19.43 ± 3.7ef |
| 1-octen-3-ol | 0.51 ± 0.1i | 7.07 ± 0.4g | 11.02 ± 0.2f | 18.69 ± 1.4c | 33.01 ± 3.7a | 3.19 ± 0.1h | 12.52 ± 0.7e | 26.73 ± 0.9b | 15.79 ± 2.1d |
| Ethyl acetate | 493.14 ± 32.2b | 835.94 ± 33.7a | 404.04 ± 32.2b | 343.52 ± 19.9c | 105.26 ± 6.1e | 884.45 ± 26.7a | 436.91 ± 9.5b | 313.14 ± 43.5c | 242.76 ± 37.3d |
| 2-pentylfuran | 1.37 ± 0.2f | 4.95 ± 0.3e | 8.58 ± 0.3d | 17.75 ± 1.8c | 24.39 ± 5.9a | 3.62 ± 0.3e | 8.51 ± 0.3c | 17.76 ± 0.3b | 14.14 ± 1.9b |
| 1-octen-3-one | 76.51 ± 10.5c | 184.13 ± 44.8b | 193.92 ± 85.4b | 197.58 ± 42.2b | 111.75 ± 38.5b | 142.87 ± 74.7b | 149.40 ± 47.2b | 116.84 ± 16.2b | 343.93 ± 62.7a |
| 2-heptanone | 0.40 ± 0.1e | 1.37 ± 0.0d | 1.85 ± 0.1d | 5.96 ± 0.7b | 8.71 ± 0.3a | 1.41 ± 0.1d | 4.50 ± 0.2c | 8.34 ± 0.1a | 5.95 ± 0.7b |
| 6-methyl-5-hepten-2-one | 0.31 ± 0.1de | 1.17 ± 0.1b | 1.86 ± 0.1ab | 1.98 ± 0.3a | 1.64 ± 0.2b | 0.46 ± 0.0d | 0.91 ± 0.0c | 1.12 ± 0.1bc | 1.08 ± 0.2bc |
| Propanoic acid | 0.26 ± 0.1d | 0.75 ± 0.2ab | 0.82 ± 0.4ab | 0.50 ± 0.2bc | 0.45 ± 0.1c | 0.29 ± 0.1d | 0.22 ± 0.1d | 0.25 ± 0.0d | 0.26 ± 0.1d |
| Hexanoic acid | 0.55 ± 0.1d | 4.03 ± 0.8c | 4.61 ± 0.5c | 16.47 ± 1.9c | 30.38 ± 8.1b | 10.63 ± 1.7c | 31.80 ± 4.5b | 133.84 ± 5.6a | 132.78 ± 12.9a |
| Sum | 638.96 ± 29.3c | 1407.49 ± 65.6b | 1276.46 ± 185.5b | 2089.68 ± 44.2a | 2526.88 ± 93.5a | 1287.9 ± 107.9b | 1529.15 ± 49.1b | 2348.74 ± 108.3a | 2522.4 ± 322.4a |
| Volatile Compound (mg/kg) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Propanol | 1.71 ± 0.2e | 10.85 ± 1.6d | 7.99 ± 0.4d | 12.74 ± 1.8d | 42.63 ± 5.6b | 22.30 ± 1.7c | 14.21 ± 0.3d | 79.83 ± 8.9a | 65.56 ± 16.5a |
| Butanal | 5.83 ± 7.9abc | 2.63 ± 0.7c | 2.88 ± 1.0c | 2.68 ± 0.4c | 6.09 ± 0.8a | 2.25 ± 0.2c | 1.30 ± 0.1c | 3.35 ± 1.10c | 4.84 ± 0.8b |
| Pentanal | 1.34 ± 0.2d | 22.14 ± 3.2b | 13.29 ± 0.4c | 13.69 ± 1.1c | 62.06 ± 9.6a | 28.91 ± 3.9b | 16.74 ± 1.2c | 31.44 ± 5.2b | 85.88 ± 15.6a |
| Hexanal | 14.72 ± 1.1f | 365.53 ± 53.8dc | 231.83 ± 6.2d | 423.67 ± 20.7c | 1454.16 ± 208.3a | 279.43 ± 41.1d | 171.20 ± 7.9e | 552.66 ± 26.3b | 1079.22 ± 196.4b |
| E-2-Pentenal | 0.09 ± 0.0d | 0.53 ± 0.1c | 0.55 ± 0.1c | 0.69 ± 0.1c | 1.81 ± 0.2b | 1.53 ± 0.3b | 1.79 ± 0.1b | 4.81 ± 1.1a | 5.02 ± 1.1a |
| Heptanal | 0.44 ± 0.1e | 1.66 ± 0.2c | 2.24 ± 0.1c | 4.05 ± 0.1b | 9.36 ± 1.2a | 1.67 ± 0.2c | 1.06 ± 0.1d | 2.94 ± 0.5c | 6.17 ± 1.8a |
| E-2-Hexenal | 0.35 ± 0.3d | 1.16 ± 0.4c | 1.39 ± 0.1c | 3.99 ± 0.2b | 8.02 ± 1.3a | 1.97 ± 0.3c | 1.44 ± 0.1c | 2.46 ± 0.3cb | 6.14 ± 1.1a |
| Octanal | 0.26 ± 0.0c | 0.65 ± 0.1b | 0.58 ± 0.0b | 0.59 ± 0.0b | 2.29 ± 0.4a | 0.51 ± 0.1b | 0.41 ± 0.0b | 0.79 ± 0.1b | 2.03 ± 0.7a |
| E-2-Heptenal | 0.20 ± 0.1f | 0.71 ± 0.1e | 0.59 ± 0.1e | 0.90 ± 0.1e | 4.68 ± 0.5b | 2.22 ± 0.4d | 2.58 ± 0.2d | 3.59 ± 0.9c | 10.15 ± 1.9a |
| Nonanal | 0.87 ± 0.1c | 1.69 ± 0.1b | 2.35 ± 0.2b | 5.22 ± 0.8b | 11.34 ± 2.3a | 1.01 ± 0.1bc | 0.70 ± 0.2c | 2.09 ± 0.6b | 1.98 ± 0.5b |
| E-2-Octenal | 0.11 ± 0.0d | 0.83 ± 0.1c | 0.54 ± 0.1c | 0.92 ± 0.1c | 6.51 ± 1.2a | 1.24 ± 0.2bc | 2.17 ± 0.1b | 2.65 ± 0.9b | 9.18 ± 1.9a |
| Benzaldehyde | 1.48 ± 0.1bc | 0.89 ± 0.1d | 1.57 ± 0.1b | 1.47 ± 0.0bc | 1.69 ± 0.1ba | 0.87 ± 0.1d | 1.13 ± 0.0bc | 1.99 ± 0.4a | 1.25 ± 0.2bc |
| E-E-2,4-Nonadienal | 0.02 ± 0.0d | 0.27 ± 0.0c | 0.15 ± 0.0c | 0.51 ± 0.0b | 1.47 ± 0.5a | 0.09 ± 0.0d | 0.08 ± 0.0d | 0.25 ± 0.1cb | 0.58 ± 0.2b |
| 1-penten-3-ol | 3.34 ± 0.0f | 15.56 ± 2.1d | 9.76 ± 0.7de | 5.65 ± 0.3e | 38.52 ± 2.9c | 45.36 ± 5.6b | 35.43 ± 1.4c | 52.23 ± 6.1b | 110.06 ± 12.4a |
| 1-pentanol | 1.43 ± 0.2f | 47.88 ± 5.9c | 25.18 ± 0.5e | 33.54 ± 1.9d | 122.76 ± 16.9a | 33.95 ± 4.2d | 25.58 ± 1.4e | 33.94 ± 3.1d | 92.28 ± 11.5b |
| Z-2-Penten-1-ol | 0.19 ± 0.0d | 0.45 ± 0.1c | 0.39 ± 0.0c | 0.56 ± 0.0c | 1.70 ± 0.0b | 0.68 ± 0.1c | 0.52 ± 0.1c | 1.18 ± 0.3b | 2.09 ± 0.3a |
| 1-hexanol | 6.82 ± 2.5f | 186.41 ± 23.0c | 139.10 ± 3.8c | 256.05 ± 36.5b | 424.06 ± 74.31a | 13.34 ± 1.3ef | 7.94 ± 2.1f | 17.89 ± 4.3e | 34.84 ± 1.3d |
| 1-octen-3-ol | 0.76 ± 0.0d | 9.59 ± 1.2c | 6.28 ± 0.5c | 7.23 ± 0.2c | 31.06 ± 4.2a | 5.15 ± 0.7c | 4.95 ± 0.5c | 7.87 ± 1.7c | 20.99 ± 3.6b |
| Ethyl acetate | 607.90 ± 20.6c | 442.25 ± 50.2d | 701.83 ± 10.2b | 406.6 ± 22.5d | 147.32 ± 13.8e | 1037.14 ± 67.9a | 1054.44 ± 48.4a | 369.97 ± 65.5d | 407.47 ± 32.6c |
| 2-pentylfuran | 1.44 ± 0.3d | 3.77 ± 0.7c | 5.14 ± 0.1c | 9.19 ± 0.6b | 24.11 ± 5.0a | 2.86 ± 0.3c | 4.35 ± 0.4c | 10.79 ± 1.9b | 8.63 ± 2.2b |
| 1-octen-3-one | 231.19 ± 57.9b | 46.35 ± 11.1e | 206.15 ± 98.9b | 203.19 ± 45.1b | 149.36 ± 22.5c | 102.36 ± 67.3d | 145.76 ± 48.1c | 498.15 ± 125.5a | 138.59 ± 53.2c |
| 2-heptanone | 0.62 ± 0.1d | 2.05 ± 0.3c | 1.51 ± 0.0c | 2.45 ± 0.3c | 8.31 ± 1.3a | 1.99 ± 0.3c | 1.82 ± 0.1c | 13.01 ± 1.8b | 1.43 ± 0.3c |
| 6-methyl-5-hepten-2-one | 0.42 ± 0.0e | 0.93 ± 0.2c | 1.09 ± 0.0bc | 1.27 ± 0.2b | 1.75 ± 0.2a | 0.59 ± 0.1de | 0.48 ± 0.0de | 0.96 ± 0.3c | 0.98 ± 0.1c |
| Propanoic acid | 0.59 ± 0.2ab | 0.22 ± 0.0b | 0.71 ± 0.3a | 0.92 ± 0.5a | 0.55 ± 0.2a | 0.41 ± 0.2ab | 0.19 ± 0.1b | 0.29 ± 0.1b | 0.43 ± 0.2ab |
| Hexanoic acid | 0.38 ± 0.1g | 5.53 ± 0.8f | 4.28 ± 0.8f | 9.12 ± 0.5e | 31.70 ± 10.4b | 15.19 ± 2.4de | 21.54 ± 2.7cd | 41.06 ± 14.5c | 106.92 ± 29.5a |
| Sum | 882.51 ± 74.1c | 1577.28 ± 222.5b | 1367.37 ± 99.5b | 1406.94 ± 110.8b | 2726.66 ± 593.9a | 1603.04 ± 194.4b | 1517.82 ± 108.5b | 1736.19 ± 197.1b | 2202.72 ± 359.3a |
| Volatile Compound (mg/kg) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Propanol | 10.44 ± 1.2f | 6.99 ± 0.52f | 7.00 ± 0.2f | 24.04 ± 0.62e | 122.14 ± 12.2ab | 107.78 ± 4.5b | 63.70 ± 9.02d | 90.48 ± 1.3c | 145.07 ± 19.6a |
| Butanal | 10.16 ± 8.1b | 23.64 ± 0.8a | 0.53 ± 0.1d | 1.80 ± 0.03cd | 4.59 ± 0.4c | 4.36 ± 0.4c | 2.14 ± 0.1c | 3.73 ± 0.4c | 2.80 ± 0.9c |
| Pentanal | 5.00 ± 0.2fe | 3.33 ± 0.5e | 2.99 ± 0.4e | 8.03 ± 0.2df | 25.22 ± 1.8c | 51.19 ± 1.7a | 24.06 ± 2.1c | 43.43 ± 0.7b | 28.53 ± 4.9c |
| Hexanal | 87.99 ± 1.9f | 117.01 ± 3.3e | 108.86 ± 0.9e | 304.92 ± 49.6d | 993.69 ± 42.4a | 705.27 ± 17.5b | 378.46 ± 40.6d | 675.37 ± 61.3b | 591.17 ± 86.4c |
| E-2-Pentenal | 0.43 ± 0.02f | 0.37 ± 0.06f | 0.20 ± 0.02f | 0.90 ± 0.2e | 1.01 ± 0.0e | 2.77 ± 0.01c | 1.98 ± 0.3d | 3.15 ± 0.2b | 6.21 ± 0.4a |
| Heptanal | 0.89 ± 0.1c | 1.26 ± 0.1c | 1.33 ± 0.0c | 2.53 ± 0.0b | 6.75 ± 0.4a | 3.05 ± 0.2b | 1.52 ± 0.1c | 3.11 ± 0.1b | 3.13 ± 0.5b |
| E-2-Hexenal | 0.11 ± 0.0f | 0.52 ± 0.1ef | 0.95 ± 0.0e | 2.39 ± 0.1b | 6.03 ± 0.4a | 1.73 ± 0.0c | 0.85 ± 0.1e | 1.49 ± 0.0cd | 1.29 ± 0.2d |
| Octanal | 0.36 ± 0.0c | 0.59 ± 0.0b | 0.51 ± 0.1b | 0.56 ± 0.1b | 1.18 ± 0.1a | 0.96 ± 0.1b | 0.38 ± 0.0c | 0.68 ± 0.1b | 0.63 ± 0.1b |
| E-2-Heptenal | 0.38 ± 0.0f | 0.60 ± 0.1ef | 0.55 ± 0.1f | 1.07 ± 0.0e | 2.94 ± 0.0d | 5.74 ± 0.3a | 3.65 ± 0.4c | 5.34 ± 0.1a | 4.61 ± 0.7b |
| Nonanal | 4.19 ± 2.9cbe | 3.71 ± 0.7d | 3.95 ± 0.5d | 9.11 ± 0.5b | 19.02 ± 1.4a | 4.03 ± 0.9c | 2.03 ± 0.4e | 2.21 ± 0.2e | 3.53 ± 0.6c |
| E-2-Octenal | 0.18 ± 0.0e | 0.42 ± 0.0e | 0.27 ± 0.0e | 0.89 ± 0.0d | 2.57 ± 0.1b | 3.75 ± 0.1a | 1.37 ± 0.3c | 2.96 ± 0.0b | 2.33 ± 0.3b |
| Benzaldehyde | 1.53 ± 0.1cbd | 1.18 ± 0.0e | 1.19 ± 0.0e | 1.36 ± 0.1de | 1.55 ± 0.1cd | 1.65 ± 0.1bc | 1.94 ± 0.3abc | 1.89 ± 0.2abc | 1.97 ± 0.2a |
| 1-pentanol | 3.39 ± 0.1f | 12.18 ± 0.3e | 11.23 ± 0.0e | 20.01 ± 0.3c | 35.48 ± 1.5ab | 39.79 ± 0.9a | 16.66 ± 1.7d | 34.89 ± 0.6b | 21.12 ± 3.3c |
| Z-2-Penten-1-ol | 0.13 ± 0.0d | 0.29 ± 0.0d | 0.36 ± 0.0d | 0.67 ± 0.0c | 1.51 ± 0.2a | 1.22 ± 0.1b | 0.69 ± 0.1c | 1.19 ± 0.1b | 1.20 ± 0.2b |
| 1-hexanol | 4.03 ± 0.0g | 93.83 ± 2.6d | 119.89 ± 2.5c | 129.73 ± 1.4a | 113.45 ± 4.4b | 13.29 ± 0.3f | 11.74 ± 1.4f | 19.95 ± 0.2e | 13.83 ± 2.1f |
| 1-octen-3-ol | 0.51 ± 0.0f | 4.03 ± 0.3e | 4.36 ± 0.1e | 8.78 ± 0.6d | 17.10 ± 0.6a | 11.02 ± 0.5b | 6.29 ± 1.1d | 10.39 ± 0.5bc | 9.34 ± 1.4c |
| 3-carene | 4.43 ± 0.2c | 5.28 ± 0.1a | 4.27 ± 0.1c | 4.77 ± 0.1a | 4.23 ± 0.2c | 4.95 ± 0.3ab | 4.42 ± 0.6bc | 4.66 ± 0.2ab | 3.48 ± 0.8b |
| 2-pentylfuran | 1.57 ± 0.0f | 3.68 ± 0.3e | 3.79 ± 0.0e | 5.32 ± 0.3d | 9.27 ± 0.5a | 7.75 ± 0.4b | 3.73 ± 0.4e | 6.56 ± 0.1c | 6.09 ± 0.9c |
| 1-octen-3-one | 479.07 ± 131.0ad | 451.29 ± 81.5ad | 545.08 ± 57.6a | 243.90 ± 228.2dc | 422.73 ± 43.0c | 473.37 ± 61.9abd | 457.52 ± 113.8abd | 364.89 ± 100.2b | 364.62 ± 131.8b |
| 2-heptanone | 1.03 ± 0.0e | 1.88 ± 0.0d | 1.59 ± 0.0d | 2.67 ± 0.5c | 4.39 ± 0.2a | 3.60 ± 0.1b | 1.92 ± 0.2d | 3.32 ± 0.1b | 2.48 ± 0.6c |
| 6-methyl-5-hepten-2-one | 0.30 ± 0.0e | 0.93 ± 0.1c | 1.21 ± 0.0b | 1.74 ± 0.1a | 1.84 ± 0.1a | 0.69 ± 0.0d | 0.77 ± 0.1d | 0.99 ± 0.1c | 0.83 ± 0.2d |
| Pentanoic acid | 6.43 ± 0.7d | 5.73 ± 0.3d | 4.30 ± 0.6d | 8.07 ± 0.8d | 14.29 ± 1.4c | 37.81 ± 2.5b | 16.45 ± 1.0c | 43.17 ± 3.3a | 44.29 ± 9.3a |
| Sum | 876.69 ± 459.8c | 1013.53 ± 548.6b | 1118.79 ± 562.1b | 1136.04 ± 524.1b | 1810.99a | 1998.57 ± 925.9a | 1388.68 ± 790.3bc | 1806.32 ± 881.9a | 2446.59 ± 1123.8a |
| Volatile Compound (mg/kg) | 5 °C | 23 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Propanol | 9.74 ± 1.5e | 6.09 ± 1.3e | 7.44 ± 0.8e | 76.05 ± 21.6c | 186.33 ± 89.3a | 39.86 ± 1.1d | 106.24 ± 0.6b | 93.23 ± 12.4bac | 165.00 ± 6.0b |
| Butanal | 0.83 ± 0.1d | 12.68 ± 0.2a | 0.45 ± 0.1d | 2.98 ± 1.0c | 6.12 ± 2.7b | 2.97 ± 0.3c | 3.89 ± 0.4c | 4.58 ± 0.7c | 4.22 ± 0.3c |
| Pentanal | 6.43 ± 1.9e | 2.76 ± 0.1f | 2.91 ± 0.1f | 19.79 ± 0.6d | 28.18 ± 14.9bd | 38.31 ± 0.5c | 47.61 ± 0.9ab | 55.68 ± 5.7a | 36.52 ± 0.3c |
| Hexanal | 119.88 ± 15.9b | 109.09 ± 2.1b | 178.17 ± 0.2b | 740.70 ± 356.7a | 1297.99 ± 699.5a | 598.03 ± 16.3a | 714.94 ± 11.8a | 954.13 ± 52.5a | 833.29 ± 118.9a |
| E-2-Pentenal | 0.39 ± 0.1d | 0.21 ± 0.1d | 0.16 ± 0.0d | 1.55 ± 0.3c | 1.30 ± 0.8c | 1.37 ± 0.1c | 2.33 ± 0.0bc | 3.02 ± 0.2ab | 3.85 ± 1.9a |
| Heptanal | 1.06 ± 0.2e | 1.60 ± 0.0d | 1.80 ± 0.1d | 6.07 ± 4.3bd | 10.57 ± 5.8a | 2.71 ± 0.0cd | 2.89 ± 0.1c | 4.51 ± 0.6c | 4.13 ± 0.2c |
| E-2-Hexenal | 0.32 ± 0.0c | 0.68 ± 0.0b | 1.62 ± 0.1b | 5.48 ± 4.4a | 9.01 ± 5.0a | 1.31 ± 0.1b | 1.43 ± 0.1b | 2.05 ± 0.1b | 1.64 ± 0.1b |
| Octanal | 0.42 ± 0.0c | 0.77 ± 0.0b | 0.56 ± 0.1b | 1.05 ± 0.6ab | 1.51 ± 0.7a | 0.76 ± 0.1b | 0.86 ± 0.1b | 1.00 ± 0.1b | 0.81 ± 0.1b |
| E-2-Heptenal | 0.32 ± 0.0f | 0.54 ± 0.0e | 0.92 ± 0.1d | 3.13 ± 0.1c | 3.42 ± 1.8c | 3.47 ± 0.1c | 5.96 ± 0.6a | 6.28 ± 0.8a | 4.91 ± 0.1b |
| Nonanal | 2.09 ± 0.5e | 6.51 ± 1.4c | 6.37 ± 0.6c | 19.93 ± 0.9b | 27.17 ± 14.5a | 3.15 ± 0.4e | 2.46 ± 0.7e | 4.86 ± 0.7cd | 6.30 ± 1.2c |
| E-2-Octenal | 0.26 ± 0.0c | 0.42 ± 0.0c | 0.47 ± 0.0c | 1.43 ± 0.3c | 2.53 ± 1.4ab | 2.35 ± 0.0b | 2.77 ± 0.3b | 3.94 ± 0.5a | 3.03 ± 0.1b |
| Benzaldehyde | 0.99 ± 0.0c | 1.28 ± 0.1bc | 1.37 ± 0.1bc | 1.96 ± 0.3ab | 2.49 ± 1.3a | 1.33 ± 0.1abc | 1.56 ± 0.1ab | 1.68 ± 0.3a | 1.87 ± 0.1a |
| 1-pentanol | 3.29 ± 0.4e | 9.15 ± 0.1d | 7.93 ± 0.2d | 22.39 ± 7.9c | 39.65 ± 20.9b | 31.69 ± 0.8bc | 38.32 ± 0.7b | 52.96 ± 4.7a | 33.12 ± 0.2bc |
| Z-2-Penten-1-ol | 0.10 ± 0.0b | 0.24 ± 0.0b | 0.38 ± 0.0b | 0.99 ± 0.4a | 1.79 ± 1.1a | 0.67 ± 0.1a | 1.08 ± 0.1a | 1.21 ± 0.2a | 1.41 ± 0.2a |
| 1-hexanol | 7.66 ± 0.9d | 89.89 ± 1.0a | 99.79 ± 0.9a | 115.44 ± 5.1a | 113.59 ± 64.2a | 31.09 ± 1.4b | 14.61 ± 0.7c | 39.29 ± 3.5b | 19.45 ± 0.7c |
| 1-octen-3-ol | 0.56 ± 0.1c | 3.42 ± 0.2b | 4.67 ± 0.1b | 11.84 ± 5.9a | 19.48 ± 11.3a | 8.71 ± 0.2a | 10.93 ± 0.6a | 13.71 ± 1.5a | 10.50 ± 0.2a |
| 3-carene | 2.44 ± 0.4ab | 4.43 ± 0.0a | 3.58 ± 0.0a | 3.45 ± 0.2a | 4.06 ± 2.3a | 3.25 ± 0.1a | 2.15 ± 0.1ab | 2.31 ± 0.2ab | 1.65 ± 0.1b |
| 2-pentylfuran | 1.41 ± 0.2c | 4.32 ± 0.1b | 3.81 ± 0.1b | 7.47 ± 4.0a | 11.12 ± 6.4a | 5.15 ± 0.2ab | 5.09 ± 0.2ab | 8.47 ± 0.8a | 7.00 ± 0.3a |
| 1-octen-3-one | 272.87 ± 7.7c | 433.28 ± 115.4b | 654.95 ± 63.8a | 447.03 ± 46.9b | 669.38 ± 278.7a | 456.13 ± 41.3b | 483.27 ± 100.9b | 409.22 ± 38.6b | 394.89 ± 68.9b |
| 2-heptanone | 1.03 ± 0.2c | 2.01 ± 0.1bc | 1.34 ± 0.1bc | 2.47 ± 0.9b | 4.82 ± 2.7a | 2.76 ± 0.1b | 3.11 ± 0.1ab | 4.55 ± 0.4a | 3.30 ± 0.1ab |
| 6-methyl-5-hepten-2-one | 0.24 ± 0.0c | 0.91 ± 0.0b | 1.15 ± 0.0b | 1.59 ± 0.8ab | 2.44 ± 1.3a | 0.86 ± 0.1b | 0.92 ± 0.1b | 1.27 ± 0.1ab | 0.99 ± 0.1b |
| Pentanoic acid | 7.49 ± 1.1e | 4.58 ± 0.4e | 5.73 ± 1.5e | 14.91 ± 1.7d | 17.04 ± 6.2cd | 23.03 ± 1.7c | 41.80 ± 3.8b | 63.79 ± 14.4a | 54.66 ± 2.5a |
| Sum | 594.06 ± 236.7c | 965.68 ± 528.1bc | 1334.75 ± 646.8b | 2159.77 ± 737.1b | 3683.16 ± 1891.3a | 1692.04 ± 786.3ab | 2018.10 ± 909.0b | 2329.37 ± 1002.9ab | 2171.88 ± 1124.3ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampofo, J.; Grilo, F.S.; Langstaff, S.; Wang, S.C. Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods 2022, 11, 3151. https://doi.org/10.3390/foods11193151
Ampofo J, Grilo FS, Langstaff S, Wang SC. Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods. 2022; 11(19):3151. https://doi.org/10.3390/foods11193151
Chicago/Turabian StyleAmpofo, Josephine, Filipa S. Grilo, Sue Langstaff, and Selina C. Wang. 2022. "Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds" Foods 11, no. 19: 3151. https://doi.org/10.3390/foods11193151
APA StyleAmpofo, J., Grilo, F. S., Langstaff, S., & Wang, S. C. (2022). Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods, 11(19), 3151. https://doi.org/10.3390/foods11193151