Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions
Abstract
:1. Introduction
2. General Information about Starch and Polyphenol Composition, Structure, and Properties and the Interaction between Starch and Phenolic Compounds
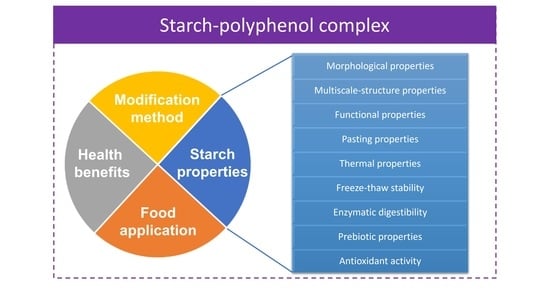
3. Properties of Starch–Polyphenol Complexes
3.1. Morphological Properties
3.2. Starch Multiscale-Structure Properties
3.2.1. Complexation Index
3.2.2. Crystallinity and Helical Structure
3.2.3. Chain Length Distribution and Molecular Composition
3.3. Swelling Power, Solubility, and Oil Absorption Capacity
3.4. Pasting Properties
3.5. Thermal Properties
3.6. Freeze–Thaw Stability
3.7. Enzymatic Digestibility
3.8. Prebiotic Properties
3.9. Antioxidant Activity
4. Current Methods for Producing Starch–Polyphenol Complexes
5. Health Effects of Starch–Polyphenol Complexes
6. Current Trends and Applications
7. Future Insights
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M.; Rizou, M.; Aldawoud, T.M.S.; Ucak, I.; Rowan, N.J. Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 2021, 110, 193–200. [Google Scholar] [CrossRef]
- Xu, L.; Ho, C.-T.; Liu, Y.; Wu, Z.; Zhang, X. Potential Application of Tea Polyphenols to the Prevention of COVID-19 Infection: Based on the Gut-Lung Axis. Front. Nutr. 2022, 9, 899842. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, L.; Zhang, R.; Liu, Y.; Wu, Z.; Zhang, X. Tea Polyphenols Prevent and Intervene in COVID-19 through Intestinal Microbiota. Foods 2022, 11, 506. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [Green Version]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Liu, S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J. Am. Coll. Nutr. 2002, 21, 298–306. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Xu, Z.; Meng, M.; Li, C.; Chen, L.; Qiu, C.; Long, J.; Jin, Z. Carbohydrate-based functional ingredients derived from starch: Current status and future prospects. Food Hydrocoll. 2022, 131, 107729. [Google Scholar] [CrossRef]
- Bede, D.; Zaixiang, L. Recent developments in resistant starch as a functional food. Starch-Stärke 2021, 73, 2000139. [Google Scholar] [CrossRef]
- Wang, S.; Wu, T.; Cui, W.; Liu, M.; Wu, Y.; Zhao, C.; Zheng, M.; Xu, X.; Liu, J. Structure and in vitro digestibility on complex of corn starch with soy isoflavone. Food Sci. Nutr. 2020, 8, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Chen, J.; Liu, C.; Dai, T.; Chen, M.; Li, T. Effects of proanthocyanidins on the pasting, rheological and retrogradation properties of potato starch. J. Sci. Food Agric. 2021, 101, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and digestibility characterisation of maize starch–caffeic acid complexes. LWT 2020, 121, 108857. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Q.; Wu, Y.; Ouyang, J. Effects of Endogenous Polyphenols in Acorn (Quercus wutaishanica Blume) Kernels on the Physicochemical Properties of Starch. Starch-Stärke 2022, 74, 2200005. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Fang, H.; Zhang, H.; Kong, X.; Wu, D.; Zheng, J.; Liu, D.; Ye, X.; Chen, S. Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins. Molecules 2020, 25, 1123. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.J.L.; Yu, J.; Zhou, W.; Liu, M.H. Effects of anthocyanins on bread microstructure, and their combined impact on starch digestibility. Food Chem. 2022, 374, 131744. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Use of Grape Peels By-Product for Wheat Pasta Manufacturing. Plants 2021, 10, 926. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch–Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Wang, S.; Guo, P. Botanical sources of starch. In Starch Structure, Functionality and Application in Foods; Springer: Berlin/Heidelberg, Germany, 2020; pp. 9–27. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L.; Wang, Z. Digestibility and supramolecular structural changes of maize starch by non-covalent interactions with gallic acid. Food Funct. 2017, 8, 720–730. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Ayua, E.O.; Nkhata, S.G.; Namaumbo, S.J.; Kamau, E.H.; Ngoma, T.N.; Aduol, K.O. Polyphenolic inhibition of enterocytic starch digestion enzymes and glucose transporters for managing type 2 diabetes may be reduced in food systems. Heliyon 2021, 7, e06245. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Moser, S.; Aragon, I.; Furrer, A.; Van Klinken, J.-W.; Kaczmarczyk, M.; Lee, B.-H.; George, J.; Hamaker, B.R.; Mattes, R.; Ferruzzi, M.G. Potato phenolics impact starch digestion and glucose transport in model systems but translation to phenolic rich potato chips results in only modest modification of glycemic response in humans. Nutr. Res. 2018, 52, 57–70. [Google Scholar] [CrossRef]
- Gothai, S.; Ganesan, P.; Park, S.-Y.; Fakurazi, S.; Choi, D.-K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, S.; Fan, S.; Ma, Y.; Li, D.; Tao, Y.; Han, Y. Encapsulation of bioactive polyphenols by starch and their impacts on gut microbiota. Curr. Opin. Food Sci. 2021, 38, 102–111. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Wang, S.; Zhou, Y. Effect of high hydrostatic pressure treatment on the formation and in vitro digestion of Tartary buckwheat starch/flavonoid complexes. Food Chem. 2022, 382, 132324. [Google Scholar] [CrossRef] [PubMed]
- Amoako, D.B.; Awika, J.M. Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chem. 2019, 285, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, H.; Sun, L.; Cao, J.; Yang, J.; Lu, M.; Wang, M. Rheological, thermal and in vitro digestibility properties on complex of plasma modified Tartary buckwheat starches with quercetin. Food Hydrocoll. 2021, 110, 106209. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Ogawa, Y.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Kong, X.; Ye, X. Co-extrusion of proanthocyanins from Chinese bayberry leaves modifies the physicochemical properties as well as the in vitro digestion of restructured rice. Food Struct. 2021, 27, 100182. [Google Scholar] [CrossRef]
- Du, J.; Yang, Z.; Xu, X.; Wang, X.; Du, X. Effects of tea polyphenols on the structural and physicochemical properties of high-hydrostatic-pressure-gelatinized rice starch. Food Hydrocoll. 2019, 91, 256–262. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Chen, J.; Dai, T.; Liu, C.; Li, T. Effect of polymeric proanthocyanidin on the physicochemical and in vitro digestive properties of different starches. LWT 2021, 148, 111713. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Ou, Y.; Zheng, B. Effect of chlorogenic acid on the structural properties and digestibility of lotus seed starch during microwave gelatinization. Int. J. Biol. Macromol. 2021, 191, 474–482. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.; Zhang, R.; Huang, L.; Jia, X.; Huang, F.; Liu, L. Physicochemical interactions between rice starch and different polyphenols and structural characterization of their complexes. LWT 2020, 125, 109227. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Hu, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Physicochemical properties, structure and in vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocoll. 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Miao, L.; Xu, Y.; Jia, C.; Zhang, B.; Niu, M.; Zhao, S. Structural changes of rice starch and activity inhibition of starch digestive enzymes by anthocyanins retarded starch digestibility. Carbohydr. Polym. 2021, 261, 117841. [Google Scholar] [CrossRef]
- Meng, S.; Ma, Y.; Sun, D.-W.; Wang, L.; Liu, T. Properties of starch-palmitic acid complexes prepared by high pressure homogenization. J. Cereal Sci. 2014, 59, 25–32. [Google Scholar] [CrossRef]
- Han, M.; Bao, W.; Wu, Y.; Ouyang, J. Insights into the effects of caffeic acid and amylose on in vitro digestibility of maize starch-caffeic acid complex. Int. J. Biol. Macromol. 2020, 162, 922–930. [Google Scholar] [CrossRef]
- He, T.; Wang, K.; Zhao, L.; Chen, Y.; Zhou, W.; Liu, F.; Hu, Z. Interaction with longan seed polyphenols affects the structure and digestion properties of maize starch. Carbohydr. Polym. 2021, 256, 117537. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Polymeric tannins significantly alter properties and in vitro digestibility of partially gelatinized intact starch granule. Food Chem. 2016, 208, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef] [Green Version]
- Kan, L.; Oliviero, T.; Verkerk, R.; Fogliano, V.; Capuano, E. Interaction of bread and berry polyphenols affects starch digestibility and polyphenols bio-accessibility. J. Funct. Foods 2020, 68, 103924. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, B.; Tan, C.P.; Ding, L.; Shao, M.; Chen, C.; Fu, X.; Huang, Q. Effect of Rosa Roxburghii juice on starch digestibility: A focus on the binding of polyphenols to amylose and porcine pancreatic α-amylase by molecular modeling. Food Hydrocoll. 2022, 123, 106966. [Google Scholar] [CrossRef]
- Kan, L.; Capuano, E.; Oliviero, T.; Renzetti, S. Wheat starch-tannic acid complexes modulate physicochemical and rheological properties of wheat starch and its digestibility. Food Hydrocoll. 2022, 126, 107459. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Lv, Y.; Li, M.; Pan, J.; Zhang, S.; Jiang, Y.; Liu, J.; Zhu, Y.; Zhang, H. Interactions between tea products and wheat starch during retrogradation. Food Biosci. 2020, 34, 100523. [Google Scholar] [CrossRef]
- Pan, J.; Li, M.; Zhang, S.; Jiang, Y.; Lv, Y.; Liu, J.; Liu, Q.; Zhu, Y.; Zhang, H. Effect of epigallocatechin gallate on the gelatinisation and retrogradation of wheat starch. Food Chem. 2019, 294, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, X.; Kong, X.; Chen, S.; Xu, E.; Liu, D.; Ogawa, Y.; Ye, X.; Tian, J. Introduction of chlorogenic acid during extrusion affects the physicochemical properties and enzymatic hydrolysis of rice flour. Food Hydrocoll. 2021, 116, 106652. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, B.; Zheng, B.; Chen, L.; Guo, Z. Effects and mechanism of high-pressure homogenization on the characterization and digestion behavior of lotus seed starch–green tea polyphenol complexes. J. Funct. Foods 2019, 57, 173–181. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Bello-Pérez, L.A. Self-assembled and assembled starch V-type complexes for the development of functional foodstuffs: A review. Food Hydrocoll. 2022, 125, 107453. [Google Scholar] [CrossRef]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose structural characteristics, methods of preparation, significance, and potential applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef] [Green Version]
- Gidley, M.J.; Bociek, S.M. Carbon-13 CP/MAS NMR studies of amylose inclusion complexes, cyclodextrins, and the amorphous phase of starch granules: Relationships between glycosidic linkage conformation and solid-state carbon-13 chemical shifts. J. Am. Chem. Soc. 1988, 110, 3820–3829. [Google Scholar] [CrossRef]
- Veregin, R.; Fyfe, C.; Marchessault, R.; Taylor, M. Characterization of the crystalline A and B starch polymorphs and investigation of starch crystallization by high-resolution carbon-13 CP/MAS NMR. Macromolecules 1986, 19, 1030–1034. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, J.; Liu, W.; Gu, Z.; Li, Z.; Cheng, L.; Li, C.; Duan, X. Sustained release of tea polyphenols from a debranched corn starch–xanthan gum complex carrier. LWT 2019, 103, 325–332. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between Amylose and Tea Polyphenols Modulates the Postprandial Glycemic Response to High-Amylose Maize Starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsuki, J. Effect of Wheat Starch Structure on Swelling Power. Cereal Chem. 1998, 75, 525–529. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. II. Waxy rice starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Radosta, S.; Kettlitz, B.; Schierbaum, F.; Gernat, C. Studies on rye starch properties and modification. Part II: Swelling and solubility behaviour of rye starch granules. Starch-Stärke 1992, 44, 8–14. [Google Scholar] [CrossRef]
- Li, M.; Pernell, C.; Ferruzzi, M.G. Complexation with phenolic acids affect rheological properties and digestibility of potato starch and maize amylopectin. Food Hydrocoll. 2018, 77, 843–852. [Google Scholar] [CrossRef]
- Van Hung, P.; Phat, N.H.; Phi, N.T.L. Physicochemical properties and antioxidant capacity of debranched starch–ferulic acid complexes. Starch-Stärke 2013, 65, 382–389. [Google Scholar] [CrossRef]
- Che, L.; Li, D.; Wang, L.; Özkan, N.; Chen, X.D.; Mao, Z. Effect of high-pressure homogenization on the structure of cassava starch. Int. J. Food Prop. 2007, 10, 911–922. [Google Scholar] [CrossRef]
- Li, G.; Niu, K.; Hou, H.; Zhang, H.; Dai, Y.; Dong, H. Effects of homogenizing pressure on mechanochemical properties of corn starch. Trans. Chin. Soc. Agric. Eng. 2017, 33, 271–277. [Google Scholar] [CrossRef]
- Shi, A.-m.; Li, D.; Wang, L.-j.; Li, B.-z.; Adhikari, B. Preparation of starch-based nanoparticles through high-pressure homogenization and miniemulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohydr. Polym. 2011, 83, 1604–1610. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Xu, B.; Jin, Z.; Tian, Y. Disruption and molecule degradation of waxy maize starch granules during high pressure homogenization process. Food Chem. 2018, 240, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Niu, M.; Xu, H. Pasting behaviors, gel rheological properties, and freeze-thaw stability of rice flour and starch modified by green tea polyphenols. LWT 2020, 118, 108796. [Google Scholar] [CrossRef]
- Sasaki, T.; Yasui, T.; Matsuki, J. Effect of amylose content on gelatinization, retrogradation, and pasting properties of starches from waxy and nonwaxy wheat and their F1 seeds. Cereal Chem. 2000, 77, 58–63. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Gu, Z.; Qiu, Y.; Cheng, L.; Hong, Y.; Li, Z. Relationship between structure and retrogradation properties of corn starch treated with 1,4-α-glucan branching enzyme. Food Hydrocoll. 2016, 52, 868–875. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, L.; Li, M.; He, X.; Hao, L.; Dai, Y. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols. Int. J. Biol. Macromol. 2019, 129, 207–213. [Google Scholar] [CrossRef]
- Li, M.; Griffin, L.E.; Corbin, S.; Neilson, A.P.; Ferruzzi, M.G. Modulating Phenolic Bioaccessibility and Glycemic Response of Starch-Based Foods in Wistar Rats by Physical Complexation between Starch and Phenolic Acid. J. Agric. Food Chem. 2020, 68, 13257–13266. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Liu, G.-Q.; Yu, F. Evaluation of Black Tea Polyphenol Extract Against the Retrogradation of Starches from Various Plant Sources. Molecules 2012, 17, 8147–8158. [Google Scholar] [CrossRef]
- Angellier-Coussy, H.; Putaux, J.-L.; Molina-Boisseau, S.; Dufresne, A.; Bertoft, E.; Perez, S. The molecular structure of waxy maize starch nanocrystals. Carbohydr. Res. 2009, 344, 1558–1566. [Google Scholar] [CrossRef]
- Cardoso, M.B.; Putaux, J.-L.; Samios, D.; da Silveira, N.P. Influence of alkali concentration on the deproteinization and/or gelatinization of rice starch. Carbohydr. Polym. 2007, 70, 160–165. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Shieh, C.-J.; Huang, S.-M.; David Wang, H.-M.; Huang, C.-Y. The effect of extrusion puffing on the physicochemical properties of brown rice used for saccharification and Chinese rice wine fermentation. Food Hydrocoll. 2019, 94, 363–370. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Zhang, F.; Fang, C.; Liu, C.; Luo, S. Freeze-thaw stability of rice starch modified by Improved Extrusion Cooking Technology. Carbohydr. Polym. 2016, 151, 113–118. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, B.; Pan, Y.; Li, X.-M.; Cheng, J.-S.; Chen, H.-Q. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chem. 2018, 264, 58–63. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, H.; Xiong, Z.; Wu, Y.; Ai, L. Effects and mechanism of sucrose on retrogradation, freeze–thaw stability, and texture of corn starch–tamarind seed polysaccharide complexes. J. Food Sci. 2022, 87, 623–635. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and characterization of highly lipophilic modified potato starch by ultrasound and freeze-thaw treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef]
- Romero Hernández, H.A.; Gutiérrez, T.J.; Bello-Pérez, L.A. Can starch-polyphenol V-type complexes be considered as resistant starch? Food Hydrocoll. 2022, 124, 107226. [Google Scholar] [CrossRef]
- Kan, L.; Capuano, E.; Fogliano, V.; Oliviero, T.; Verkerk, R. Tea polyphenols as a strategy to control starch digestion in bread: The effects of polyphenol type and gluten. Food Funct. 2020, 11, 5933–5943. [Google Scholar] [CrossRef]
- Fei, Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X. Effects of Oolong Tea Polyphenols, EGCG, and EGCG3″Me on Pancreatic α-Amylase Activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Xu, H.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef]
- Xu, T.; Zhong, Y.; Chen, Q.; Wu, L.; Ji, S.; Yang, B.; Zhang, Y.; Shen, J.; Lu, B. Modulating the digestibility of cassava starch by esterification with phenolic acids. Food Hydrocoll. 2022, 127, 107432. [Google Scholar] [CrossRef]
- Xu, T.; Li, X.; Ji, S.; Zhong, Y.; Simal-Gandara, J.; Capanoglu, E.; Xiao, J.; Lu, B. Starch modification with phenolics: Methods, physicochemical property alteration, and mechanisms of glycaemic control. Trends Food Sci. Technol. 2021, 111, 12–26. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.; Ashaolu, J.; Adeyeye, S. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.-K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Phenolic compounds: Evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol. Nutr. Food Res. 2008, 52, 53–61. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Xiao, P.; Li, C.; Zhou, H.; Liu, D. Informative title: Incorporation of finger millet affects in vitro starch digestion, nutritional, antioxidative and sensory properties of rice noodles. LWT 2021, 151, 112145. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional study of raw and cooked blue maize flour: Starch digestibility, total phenolic content and antioxidant activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- Ou, S.; Yang, A.L.A. A study on synthesis of starch ferulate and its biological properties. Food Chem. 2001, 74, 91–95. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Xie, F.; Li, L.; Bai, G. Modulating the in vitro digestibility and predicted glycemic index of rice starch gels by complexation with gallic acid. Food Hydrocoll. 2019, 89, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, X.; Guo, Z.; Zheng, B.; Zhang, Y. Insights into the multi-scale structural properties and digestibility of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. Food Chem. 2021, 361, 130171. [Google Scholar] [CrossRef]
- Reddy, C.K.; Son, S.Y.; Lee, C.H. Effects of pullulanase debranching and octenylsuccinic anhydride modification on the structural properties of maize starch-green tea extract complexes. Food Hydrocoll. 2021, 115, 106630. [Google Scholar] [CrossRef]
- Oladele, A.K.; Duodu, K.G.; Emmambux, N.M. Hydrolysis and antioxidant activity of starch modified with phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Carbohydr. Polym. 2020, 240, 116291. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Li, Z.; Gao, Y.; Cui, S.W.; Wang, T.; Qiu, J. Diverse effects of rutin and quercetin on the pasting, rheological and structural properties of Tartary buckwheat starch. Food Chem. 2021, 335, 127556. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Tasman-Jones, C.; Englyst, H.; Harris, P.J. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr. Cancer 2000, 36, 230–237. [Google Scholar] [CrossRef]
- Toutounji, M.R.; Farahnaky, A.; Santhakumar, A.B.; Oli, P.; Butardo, V.M.; Blanchard, C.L. Intrinsic and extrinsic factors affecting rice starch digestibility. Trends Food Sci. Technol. 2019, 88, 10–22. [Google Scholar] [CrossRef]
- Panyathep, A.; Chewonarin, T. Inhibitory effect of a gamma-oryzanol-rich fraction from purple rice extract on lipopolysaccharide-induced metastasis in human colon cancer cells. J. Food Biochem. 2020, 44, e13487. [Google Scholar] [CrossRef]
- Mhillaj, E.; Catino, S.; Miceli, F.M.; Santangelo, R.; Trabace, L.; Cuomo, V.; Mancuso, C. Ferulic Acid Improves Cognitive Skills Through the Activation of the Heme Oxygenase System in the Rat. Mol. Neurobiol. 2018, 55, 905–916. [Google Scholar] [CrossRef]
- Alam, M. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S.; Yanase, E. Slow starch digestion in the rice cooked with adzuki bean: Contribution of procyanidins and the oxidation products. Food Res. Int. 2019, 119, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yamuangmorn, S.; Prom, U.T.C. The Potential of High-Anthocyanin Purple Rice as a Functional Ingredient in Human Health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Nyambe-Silavwe, H.; Williamson, G. Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: A randomised, controlled, single-blind, cross-over intervention. Br. J. Nutr. 2016, 116, 443–450. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Foods 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: Its quality attributes and in vitro digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Arlorio, M.; Coïsson, J.D. Spray-dried polyphenolic extract from Italian black rice (Oryza sativa L.; var. Artemide) as new ingredient for bakery products. Food Chem. 2018, 269, 603–609. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Bertolino, M.; Barbosa-Pereira, L.; Ben Haj Kbaier, H.; Bouzouita, N.; Zeppa, G. Almond Hull as a Functional Ingredient of Bread: Effects on Physico-Chemical, Nutritional, and Consumer Acceptability Properties. Foods 2022, 11, 777. [Google Scholar] [CrossRef]
- Pico, J.; Corbin, S.; Ferruzzi, M.G.; Martinez, M.M. Banana flour phenolics inhibit trans-epithelial glucose transport from wheat cakes in a coupled in vitro digestion/Caco-2 cell intestinal model. Food Funct. 2019, 10, 6300–6311. [Google Scholar] [CrossRef]
- Morina, F.; Hirota, S.; Takahama, U. Contribution of amylose-procyanidin complexes to slower starch digestion of red-colored rice prepared by cooking with adzuki bean. Int. J. Food Sci. Nutr. 2020, 71, 715–725. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S.; Morina, F. Procyanidins in rice cooked with adzuki bean and their contribution to the reduction of nitrite to nitric oxide ((•)NO) in artificial gastric juice. Int. J. Food Sci. Nutr. 2020, 71, 63–73. [Google Scholar] [CrossRef]
- Joymak, W.; Ngamukote, S.; Chantarasinlapin, P.; Adisakwattana, S. Unripe Papaya By-Product: From Food Wastes to Functional Ingredients in Pancakes. Foods 2021, 10, 615. [Google Scholar] [CrossRef]
- Han, H.M.; Koh, B.K. Effect of phenolic acids on the rheological properties and proteins of hard wheat flour dough and bread. J. Sci. Food Agric. 2011, 91, 2495–2499. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Xu, J.; Dai, T.; Chen, J.; He, X.; Shuai, X.; Liu, C.; Li, T. Effects of Three Types of Polymeric Proanthocyanidins on Physicochemical and In Vitro Digestive Properties of Potato Starch. Foods 2021, 10, 1394. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Li, X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Wu, L.; Che, L.; Chen, X.D. Antiretrogradation in cooked starch-based product application of tea polyphenols. J. Food Sci. 2014, 79, E1984–E1990. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef]

| Complex | Analysis Method | Key Findings | Reference |
|---|---|---|---|
| Maize starch + caffeic acid | SEM | Maize starch had a spherical, smooth structure with a few microscopic pores, whereas the complexes had a rough surface and an angular form. More holes were detected when the concentration of caffeic acid was increased. | [15] |
| Buckwheat starch + flavonoid solution | SEM | Natural buckwheat had an elliptical structure with a smooth surface. During the treatment of the complex starch, small holes appeared, but the original morphology of the starch granules was retained. | [34] |
| Maize starch + tannin from sorghum | FE-SEM | PA-complexed samples had clearly identifiable granules and granule fragments. However, these granules showed signs of significant enzyme damage from within and a spongy appearance. | [35] |
| Buckwheat starch + quercetin | SEM | Quercetin bound to starch granules, producing a smoother and more compact grain structure. However, when processed at high temperatures, this structure was easily broken. | [36] |
| Rice starch + proanthocyanin from Chinese bayberry leaves | SEM, digital camera | Rice grains increased in color intensity with increasing concentrations of Chinese bayberry leaf extract during processing. Grain texture was not significantly affected. | [37] |
| Rice starch + tea polyphenols | PLM, SEM | Under 400 MPa, starch granules’ morphological and birefringent characteristics tended to deteriorate as tea polyphenol concentration increased, and more structural damage to starch granules occurred. | [38] |
| Potato starch + proanthocyanins | SEM | The porosity of the starch complex gradually increased with increasing proanthocyanin concentration. When compared with natural potato starch, the microstructure of the grain was smaller and denser. | [14] |
| Rice, potato, pea starch + polymeric proanthocyanidins | SEM | The addition of polymeric proanthocyanidins changed the surface structure of the starch; specifically, the volume and number of pores were increased, resulting in a more stretched microstructure. | [39] |
| Lotus seed starch + chlorogenic acid | SEM | The interaction between lotus seed starch and chlorogenic acid made the granule surface rougher. | [40] |
| Lotus seed starch + green tea polyphenols | SEM, CLSM, laser scattering measurement | Ultrasound-assisted processing caused the complex starch to be broken into smaller pieces. A rough, homogeneous net of microparticles also appeared to cover the surface. CLSM and laser scattering measurements were used to confirm this result. | [41] |
| Starch | Research Methodology | Characterization Method | Key Findings | Crystalline Structure | Reference |
|---|---|---|---|---|---|
| Maize starch | Longan seed polyphenols (LSPs): starch (approx. 61% amylose) = 1:5 (w/w) | FT-IR, WXRD | Maize starch could interact with LSPs through non-covalent interaction. After complexation, new diffraction peaks were seen at 2θ of 7.5°, 12.7°, and 20.1°. | V-type | [47] |
| Mixed with caffeic acid (0.2–1.0%, w/w) in 50 mL of ethanol solution (30%) | XRD, FT-IR | Caffeic acid affects maize starch crystal degree rather than crystal type. The primary force between maize starch and caffeic acid was non-covalent contact. | A-type | [15] | |
| High-amylose, normal, waxy maize starch mixed with 500 mg caffeic acid | 1H-NMR, XRD, FT-IR, Iodine binding | The complexation index reduced as the amylose concentration increased. After the incorporation of caffeic acid, the crystallinity was a downward trend, and a V-type crystal formed. | V-type | [46] | |
| Normal and waxy maize starch complexed with tannins from sorghum | Iodine binding, XRD, FE-SEM | H-bonding helps/stabilizes the semi-crystalline V-complexes formed by high-molecular-weight proanthocyanidins and amylose. Amorphous complexes arise when H-bonding is restricted. | V-type | [35] | |
| Tartary Buckwheat starch | 10% flavonoid solution | XRD, FT-IR | Under high hydrostatic pressure treatment, complexation does not change the crystalline type but can increase crystallinity. | A-type | [34] |
| Quercetin | XRD, FT-IR | New scattered peaks appeared at 2θ of approximately 12.5°, 13.6°, 19.2°, 21.0°, 23.9°, and 27.3°. Quercetin interacts with starch through non-covalent bonds such as hydrogen bonds during gelatinization. Intermolecular H–H interaction is likely to occur between the two components. | V-type | [36] | |
| Wheat starch | Tannic acid | XRD | Wheat starch’s relative crystallinity is improved by complexation with tannic acid. In all samples containing tannic acid, a new wide peak was identified at a 2θ of 20°. | V-type | [52] |
| Tea polyphenols | XRD, FT-IR | Tea polyphenol–wheat starch complexes show broader O-H stretching and C-O-H bending vibrations. Polyphenols from tea likely create hydrogen bonds with wheat starch. | B-type, V-type | [54] | |
| Epigallocatechin gallate (EGCG) | XRD, FT-IR, Raman spectroscopy | FT-IR and Raman spectroscopy revealed that EGCG and wheat starch form hydrogen bonds. The positions of the diffraction peaks were unaffected by the addition of EGCG; however, their intensity was reduced. | B-type | [55] | |
| Rice starch | Chlorogenic acid | WXDR, FT-IR | Chlorogenic acid enhances the crystallinity of rice starch and mainly interacts with the starch through hydrogen bonds. | V-type | [56] |
| Proanthocyanidins from Chinese bayberry leaves | XRD | A new diffraction peak was found at a 2θ of 28°, indicating that Chinese bayberry proanthocyanidins and rice flour may interact in a new way. The crystallinity degree is higher in untreated rice flour. | V-type | [37] | |
| Anthocyanins from black rice | Iodine binding, XRD, FT-IR, Molecular dynamics, and Molecular docking | Anthocyanins complex with rice starch through a non-covalent connection. A peak observed for rice starch at 17° vanished after anthocyanins were added, indicating a change in the crystalline structure. The broad peak at 3400 cm−1 was reduced in intensity, indicating a decrease in the amount of OH groups and bonds among starch molecules. | A-type | [44] | |
| Tea polyphenols with high-hydrostatic-pressure-gelatinized rice starch | 13C CP/MAS NMR spectroscopy, XRD | A combination of B + V-type structure was observed in tea polyphenol-treated starch (600 MPa). Disruption of rice starch granules increased with increasing pressure level during pressurization, whereas relative crystallinity decreased. | A-type, B-type, V-type | [38] | |
| Potato starch | Proanthocyanins | FT-IR, XRD | The intermolecular hydrogen-bonding interaction between proanthocyanin and potato starch prevented retrogradation of the starch. After treatment, a V-type diffraction peak replaced the B-type peak. | V-type | [14] |
| Grape seed proanthocyanidins (GSP) (0.0–5.0%, based on starch weight) | XRD, FT-IR | There were no distinct peaks in GSP-potato starch complexes, indicating that the long-range crystalline structure had been broken. With the addition of 3.0–5.0% GSP, two additional peaks were discovered at around 2θ of 34° and 37.4°, which indicated the formation of a novel crystal structure. | New crystal structure | [17] | |
| Rice, potato, pea starch | Polymeric proanthocyanidins (PPC, purity ≥ 95%) isolated from grape seeds | FT-IR, XRD | Short- and long-term retrogradation of three starches were both inhibited by PPC. The long-range organized structure of starch was mostly changed through hydrogen bonding. | V-type | [44] |
| Lotus seed starch | Chlorogenic acid (5%) under microwave gelatinization and hydrotreatment | 13C CP/MAS NMR spectroscopy, FT-IR | The V-type complex becomes dominant after complete gelatinization (85 °C), cooling, and recrystallization. It acts as a barrier to water and digestive enzymes and inhibits starch enzymatic hydrolysis. | V-type B-type C-type | [40] |
| Green tea polyphenols | WXRD | When the pressure was increased to 150 MPa, a V-type crystal structure was formed. Starch fragments took on a “flower-like” form with spherical crystals thickly dispersed throughout their surfaces. LS-GTP complexes exhibited a non-inclusive surface structure with a C-type crystalline structure. | V-type C-type | [57] |
| Starch | Complexation Condition | Pasting Properties * | Thermal Properties ** | Swelling Power (%) | Solubility (g/g) | Freeze–Thaw Stability | Ref. |
|---|---|---|---|---|---|---|---|
| Corn starch | Native | PV: 4242 BV: 2016 SV: 1944 FV: 4170 | - | - | - | - | [74] |
| High tannin: 5-20% | PV: 4515.6–4720.8 BV: 1929.6–2162.4 SV: 1636.8–1819.2 FV: 4144.8–4428 | - | - | - | - | ||
| Rice starch | Native | PV: 2577 ± 3 BV: 438 ± 7 SV: 619 ± 13 PT: 79.13 ± 0.71 | To = 62.23 ± 0.09 Tp = 69.47 ± 0.09 Tc = 78.03 ± 0.17 ΔH = 10.32 ± 0.26 | 9.55 ± 0.22% at 85 °C | 6.22 ± 0.35 at 85 °C | - | [42] |
| Ferulic acid: 5–20% | PV: 2383–2605 BV: 1112–1506 SV: −434–−240 PT: 86.70–89.30 | To = 61.17 ± 0.05–61.37 ± 0.57 Tp = 68.23 ± 0.09–68.37 ± 0.25 Tc = 76.50 ± 0.14–76.80 ± 0.33 ΔH = 9.62 ± 0.22–10.00 ± 0.05 | 7.84 ± 0.03– 9.39 ± 0.12% at 85 °C | 7.98 ± 0.3 –17.18 ± 0.38 at 85 °C | - | ||
| Gallic acid: 5–20% | PV: 2338–2584 BV: 1020–1515 SV: −905–−256 PT: 84.58–89.60 | To = 48.57 ± 0.05–59.23 ± 0.60 Tp = 55.83 ± 0.05–66.70 ± 0.50 Tc = 63.80 ± 0.57–76.13 ± 0.40 ΔH = 7.54 ± 0.27–11.20 ± 0.25 | 8.31 ± 0.27–9.91 ± 0.12 at 85 °C | 9.27 ± 0.2–21.71 ± 0.46 at 85 °C | - | ||
| Quercetin: 5–20% | PV: 2779–3301 BV: 464–811 SV: -20–491 PT: 76.98–80.08 | To = 62.33 ± 0.12–62.53 ± 0.12 Tp = 69.60 ± 0.33–69.80 ± 0.33 Tc = 77.70 ± 0.16–78.30 ± 0.37 ΔH = 9.19 ± 0.54–10.06 ± 0.03 | 9.19 ± 0.12–9.33 ± 0.05 at 85 °C | 5.56 ± 0.2–6.00 ± 0.05 at 85 °C | - | ||
| Rice starch | Native | PV: 3440 BV: 1923.67 SV: 1449 FV: 2965.33 | Tp = 68.59 ± 0.37 ΔH = 12.39 ± 0.32 | - | - | ~ 35% | [75] |
| Green tea polyphenols | PV: 2538–2963 BV: 1484.7–1744.3 SV: 652.00–964.00 FV: 1705.3–2778.8 | Tp = 56.28 ± 0.33–64.78 ± 0.10 ΔH = 5.56 ± 0.04–12.39 ± 0.32 | - | - | ~ 0.2–22% | ||
| Maize starch | Native | PV: 3091 ± 23 BV: 1237 ± 12 SV: 1739 ± 16 FV: 3594 ± 17 | To = 59.98 ± 0.95 Tc = 73.10 ± 1.00 Tp = 65.35 ± 0.46 ΔH = 17.12 ± 0.26 | ~ 17% at 95 °C | ~ 14% at 95 °C | ~ 60% (5 cycles) | [15] |
| Caffeic acid: 0.0–1.0% | PV: 2197–2992 BV: 1184–1452 SV: 773–1722 FV: 1599–3529 | To = 60.04 ± 0.42–64.94 ± 0.18 Tc = 73.75 ± 0.57–77.01 ± 0.09 Tp = 65.35 ± 0.40–66.98 ± 0.25 ΔH = 13.46 ± 0.54–16.56 ± 0.32 | ~ 14–17% at 95 °C | ~ 8.0–12.5% at 95 °C | ~ 40–55% (5 cycles) | ||
| Potato starch | Native starch | PV: 3496 ± 32.31 BV: 1688 ± 18.99 SV: 449 ± 11.23 FV: 2137 ± 21.23 PT: 63.50 ± 0.16 | To = 58.87 ± 0.52 Tp = 62.94 ± 0.46 Tc = 68.32 ± 0.37 ΔH = 11.39 ± 0.37 | - | - | - | [14] |
| Proanthocyanidins: 2.5–7.5% | PV: 1325–2322 BV: 120–790 SV: 318–415 FV: 1620–1850 PT: 68–69 | To = 59.98 ± 0.47–61.40 ± 0.30 Tp = 64.17 ± 0.37–65.99 ± 0.44 Tc = 70.29 ± 0.38–71.80 ± 0.50 ΔH = 9.31 ± 0.09–10.03 ± 0.19 | - | - | - | ||
| Rice starch | Native | PV: 2388.67 ± 17.46 BV: 944 ± 3.74 SV: 1114 ± 3.56 FV: 2558.67 ± 22.95 | To = 65.61 ± 0.47 Tp = 71.71 ± 0.63 Tc = 80.99 ± 0.55 ΔH = 15.98 ± 0.20 | - | - | - | [37] |
| Chinese bayberry leaf extract: 0.1–2% | PV: 341.33–427.33 BV: 127.00–223.00 SV: 408.00–424.00 FV: 609.67–639.67 | To = 58.49 ± 0.10–61.28 ± 0.87 Tp = 66.00 ± 1.00–67.82 ± 0.28 Tc = 72.61 ± 0.72–77.97 ± 0.51 ΔH = 9.59 ± 0.07–11.41 ± 0.04 | - | - | - | ||
| Soluble starch | Native | - | To = 59.95 ± 0.23 Tp = 65.09 ± 0.58 Tc = 73.12 ± 1.10 ΔH = 17.07 ± 1.31 | - | - | - | [43] |
| Lotus leaf flavonoids crude extract | - | To = 60.81 ± 0.78–61.63 ± 0.45 Tp = 65.43 ± 0.10–67.09 ± 0.58 Tc = 73.27 ± 0.37–73.91 ± 0.33 ΔH = 10.60 ± 0.71–13.23 ± 0.63 | - | - | - | ||
| Potato starch | Native | PV: 1600 ± 2.9 PT: 71.6 ± 0.80 BV: 691 ± 6.2 SB: −519 ± 5.1 | - | - | - | - | [67] |
| Caffeic acid | PV: 951 ± 71 PT: 66.0 ± 1.5 BV: 667 ± 7.0 SB: −617 ± 27 | - | - | - | - | ||
| Gallic acid | PV: 461 ± 13 PT: 68.5 ± 0.057 BV: 242 ± 8.1 SB: −200 ± 9.0 | - | - | - | - | ||
| Ferulic acid | PV: 829 ± 21 PT: 69.6 ± 0.50 BV: 598 ± 16 SB: −561 ± 13 | - | - | - | - | ||
| Maize amylopectin | Native | PV: 7070 ± 54 PT: 67.7 ± 0.68 BV: 4780 ± 67 SB: −4430 ± 63 | - | - | - | - | |
| Caffeic acid | PV: 204 ± 2.6 PT: 50.9 ± 1.1 BV: 145 ± 2.5 SB: −132 ± 2.1 | - | - | - | - | ||
| Gallic acid | PV: 281 ± 2.5 PT: 50.4 ± 0.076 BV: 228 ± 2.5 SB: −212 ± 3.6 | - | - | - | - | ||
| Ferulic acid | PV: 206 ± 3.2 PT: 50.3 ± 0.029 BV: 160 ± 4.6 SB: −147 ± 4.6 | - | - | - | - | ||
| Potato starch | Native | - | To = 49.8 ± 0.2 Tp = 84.5 ± 0.4 Tc = 99.6 ± 0.1 ΔH = 992.4 ± 0.3 | - | - | - | [78] |
| Tea polyphenols | - | To = 60.4 ± 0.1 Tp = 88.0 ± 0.3 Tc = 102.2 ± 0.4 ΔH = 1353.3 ± 0.1 | - | - | - |
| Starch | Polyphenol | Type of Modification | Modification Method | References |
|---|---|---|---|---|
| Rice starch | Tea polyphenols | High hydrostatic pressure treatment |  | [38] |
| Lotus seed starch | Green tea polyphenols | High-pressure homogenization |  | [57] |
| Lotus seed starch | Green tea polyphenols | Ultrasound-microwave synergistic processing |  | [41] |
| Lotus seed starch | Chlorogenic acid | Microwave irradiation |  | [104] |
| Tartary buckwheat starch | Tartary buckwheat flavonoids | - |  | [107] |
| Tartary buckwheat starch | Quercetin | Plasma Pre-gelatinization |  | [36] |
| Rice starch | Ferulic acid, gallic acid, quercetin | - |  | [42] |
| Rice starch | Anthocyanins | - |  | [44] |
| Maize starch | Grape pomace and sorghum bran | pH-based modification (alkaline condition) |  | [106] |
| Maize starch | Green tea extract | Enzymatic modification (Pullulanase debranching or octenylsuccinic anhydride) |  | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, T.V.; Kusumawardani, S.; Kunyanee, K.; Luangsakul, N. Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions. Foods 2022, 11, 3384. https://doi.org/10.3390/foods11213384
Ngo TV, Kusumawardani S, Kunyanee K, Luangsakul N. Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions. Foods. 2022; 11(21):3384. https://doi.org/10.3390/foods11213384
Chicago/Turabian StyleNgo, Tai Van, Sandra Kusumawardani, Kannika Kunyanee, and Naphatrapi Luangsakul. 2022. "Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions" Foods 11, no. 21: 3384. https://doi.org/10.3390/foods11213384
APA StyleNgo, T. V., Kusumawardani, S., Kunyanee, K., & Luangsakul, N. (2022). Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions. Foods, 11(21), 3384. https://doi.org/10.3390/foods11213384