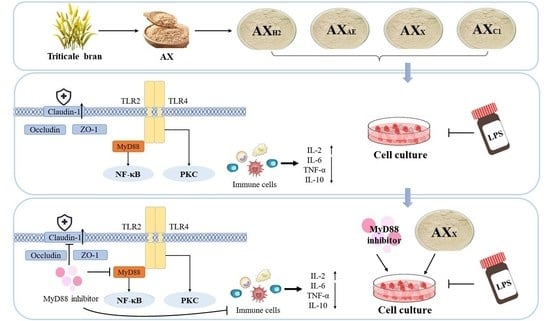
Different Structures of Arabinoxylan Hydrolysates Alleviated Caco-2 Cell Barrier Damage by Regulating the TLRs/MyD88/NF-κB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Arabinoxylan Hydrolysates
2.3. Structural Characterisations of Arabinoxylan Hydrolysates
2.3.1. Molecular Weight Measurement
2.3.2. Monosaccharide Composition Measurement
2.3.3. 1H NMR Analysis
2.3.4. FT-IR Analysis
2.3.5. Ferulic Acid Determination
2.4. Establishment of the Cell Model
2.4.1. Cell Culture
2.4.2. Treatment of Cells with Arabinoxylan Hydrolysates
2.4.3. Treatment of Cells with MyD88 Inhibitor
2.5. Determination of Epithelial Monolayer Resistance
2.6. Assessment of Protein Expression by Western Blot
2.7. Quantification of Gene Expression Using Real-Time PCR
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Statistical Analysis
3. Results
3.1. Structural Characterisations of Arabinoxylan Hydrolysates
3.1.1. Molecular Weight and Degree of Substitution of Arabinoxylan Hydrolysates
3.1.2. 1H NMR Analysis of Arabinoxylan Hydrolysates
3.1.3. FT-IR Spectrum Analysis of Arabinoxylan Hydrolysates
3.1.4. Ferulic Acid Content Analysis
3.2. Regulating Effects of AXH on the Intestinal Barrier in the Caco-2 Model
3.2.1. Transepithelial Electrical Resistance Measurement
3.2.2. Tight Junction Protein Expression in Caco-2 Cells
3.2.3. Signaling Pathway in Caco-2 Cells
3.2.4. Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, J.; Qu, H.; Shu, D. Crosstalk between bioactive peptide and intestinal barrier in gut homeostasis. Curr. Protein Pept. Sci. 2015, 16, 604–612. [Google Scholar] [CrossRef]
- Thadi, A.; Patwa, V.; Joshi, A.; Foss, J.A.; Eddy, E.P.; Palejwala, V.A.; Shailubhai, K. Plecanatide and dolcanatide, guanylate cyclase-C agonists, attenuate paracellular permeability and enhance normal localization of tight junctional proteins to maintain intestinal barrier function. Gastroenterology 2017, 152, S506. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Wells, J.; Cani, P.D.; Ródenas, C.L.G.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [Green Version]
- Cario, E.; Gerken, G.; Podlsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Kaminsky, L.; Sadi, R.A.; Ma, T. Lactobacillus acidophilus causes enhancement of the intestinal tight junction barrier by a toll-like receptor-2-dependent increase in occludin. J. Allergy Clin. Immunol. 2022, 149, AB99. [Google Scholar] [CrossRef]
- Bruning, E.E.; Coller, J.K.; Wardill, H.R.; Bowen, J.M. Site-specific contribution of Toll-like receptor 4 to intestinal homeostasis and inflammatory disease. J. Cell. Physiol. 2021, 236, 877–888. [Google Scholar] [CrossRef]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic acid enhances tight junctions and ameliorates inflammation in Salmonella typhimurium-induced diarrhea in mice via the TLR4/NF-kappaB and MAPK pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappaB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, antioxidant, and hypoglycemic activities of arabinoxylans extracted by multiple methods from triticale. Antioxidants 2019, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Wiele, T.V.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2014, 51, 178–194. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, W.; Qu, W.; Wang, K. Arabinoxylan rice bran (MGN-3/Biobran) alleviates radiation-induced intestinal barrier dysfunction of mice in a mitochondrion-dependent manner. Biomed. Pharmacother. 2020, 124, 109855. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; Wilms, E.; Truchado, P.; Vargas, R.V.; Pieper, D.H.; Jáuregui, R.; Marzorati, M.; Wiele, T.; Possemiers, S.; et al. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: A randomized controlled trial: Arabinoxylans in gut barrier. Clin. Nutr. 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Zhao, J. Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylan hydrolyzates as immunomodulators in Caco-2 and HT-29 colon cancer cell lines. Food Funct. 2017, 8, 220–231. [Google Scholar] [CrossRef]
- Bijalwan, V.; Ali, U.; Kesarwani, A.K.; Yadav, K.; Mazumder, K. Hydroxycinnamic acid bound arabinoxylans from millet brans-structural features and antioxidant activity. Int. J. Biol. Macromol. 2016, 88, 296–305. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Production of structurally diverse wheat arabinoxylan hydrolyzates using combinations of xylanase and arabinofuranosidase. Carbohyd. Polym. 2015, 132, 452–459. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Yang, T.; Chen, D.; Xiao, Y.; Qin, W.; Wu, D.; Zhang, Q.; Lin, D.; Liu, Y.; et al. Interactive effects of molecular weight and degree of substitution on biological activities of arabinoxylan and its hydrolysates from triticale bran. Int. J. Biol. Macromol. 2021, 166, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.; Liu, W.; Su, Y.; Han, Q.; Zhao, L.; Zhang, Q.; Lin, D.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, L.; Tuomainen, P.; Virkki, L.; Tenkanen, M. Molecular characterization and solution properties of enzymatically tailored arabinoxylans. Int. J. Biol. Macromol. 2011, 49, 963–969. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Antioxidant capacity of arabinoxylan oligosaccharide fractions prepared from wheat aleurone using Trichoderma viride or Neocallimastix patriciarum xylanase. Food Chem. 2015, 167, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liang, F.; Liu, K.; Qaiser, S.; Pan, S.; Xu, X. Structure characteristics for intestinal uptake of flavonoids in Caco-2 cells. Food Res. Int. 2018, 105, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, T.; Li, Y.; Gao, Z.; Zhang, K.; Song, J.; Xiao, J.; Cao, Y. Granny Smith apple procyanidin extract upregulates tight junction protein expression and modulates oxidative stress and inflammation in lipopolysaccharide-induced Caco-2 cells. Food Funct. 2018, 9, 3321–3329. [Google Scholar] [CrossRef]
- Song, J.; Chen, D.; Pan, Y.; Shi, X.; Liu, Q.; Lu, X.; Xu, X.; Chen, G.; Cai, Y. Discovery of a novel MyD88 inhibitor M20 and its protection against sepsis-mediated acute lung injury. Front. Pharmacol. 2021, 12, 775117. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Ouyang, Y.; He, Y.; Xiao, J.; Zhang, L.; Feng, N. Effect of catechin on dietary AGEs absorption and cytotoxicity in Caco-2 cells. Food Chem. 2021, 355, 129574. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Sui, Y.; Yang, Y.; Dong, L.; Xie, B.; Sun, Z. Inhibition of advanced glycation endproduct formation by lotus seedpod oligomeric procyanidins through RAGE–MAPK signaling and NF-κB activation in high-fat-diet rats. J. Agric. Food Chem. 2015, 63, 6989–6998. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Lange, I.G.; Daxenberger, A.; Meyer, H.H.D. Tissue-specific expression pattern of estrogen receptors (ER): Quantification of ERα and ERβ mRNA with real-time RT-PCR. APMIS 2001, 109, 345–355. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, W.; Degroote, J.; Possemiers, S.; Chen, D.; de Smet, S.; Michiels, J. Arabinoxylan in wheat is more responsible than cellulose for promoting intestinal barrier function in weaned male piglets. J. Nutr. 2015, 145, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhou, D.; Xiao, H.; Fu, X.; Kong, Q.; Zhu, C.; Han, Z.; Mou, H. Marine-derived uronic acid-containing polysaccharides: Structures, sources, production, and nutritional functions. Trends Food Sci. Technol. 2022, 122, 1–12. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Kostálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohyd. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Henry, K.C.J.; Napper, S.; Brien, C.; Jones, N.L.; Sherman, P.M. Protein kinase C delta signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef] [Green Version]
- Itallie, C.M.V.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-cell junctions organize structural and signaling networks. Csh. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Jo, H.; Hwang, D.; Kim, J.K.; Lim, Y.H. Oxyresveratrol improves tight junction integrity through the PKC and MAPK signaling pathways in Caco-2 cells. Food Chem. Toxicol. 2017, 108, 203–213. [Google Scholar] [CrossRef]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; Vos, P. Toll-like receptor 2 activation by beta2→1-fructans protects barrier function of T84 human intestinal epithelial cells in a chain length-dependent manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardill, H.R.; Gibson, R.J.; Sebille, Y.Z.A.V.; Secombe, K.R.; Coller, J.K.; White, I.A.; Manavis, J.; Hutchinson, M.R.; Staikopoulos, V.; Logan, R.M.; et al. Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol. Cancer Ther. 2016, 15, 1376–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paesani, C.; Degano, A.L.; Zalosnik, M.I.; Fabi, J.P.; Perez, G.T. Enzymatic modification of arabinoxylans from soft and hard Argentinian wheat inhibits the viability of HCT-116 cells. Food Res. Int. 2021, 147, 110466. [Google Scholar] [CrossRef] [PubMed]
- Nighot, M.; Sadi, R.A.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef] [Green Version]
- Yuki, T.; Yoshida, H.; Akazawa, Y.; Komiya, A.; Sugiyama, Y.; Inoue, S. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J. Immunol. 2011, 187, 3230–3237. [Google Scholar] [CrossRef]







| Samples | Monosaccharide Composition (Molar Ratio) | A/X | Average Molecular Weight (Da) | Polydispersity Index (Mw/Mn) | |||
|---|---|---|---|---|---|---|---|
| Xylose Arabinose Glucose Galactose | |||||||
| AXH2 | 1.00 | 0.15 | 0.04 | 0.05 | 0.15 | 7.47 × 103 | 1.81 |
| AXAE | 1.00 | 0.97 | 0.02 | 0.05 | 0.97 | 2.67 × 103 | 1.24 |
| AXX | 1.00 | 1.17 | 0.02 | 0.07 | 1.17 | 6.43 × 105 | 2.28 |
| AXC1 | 1.00 | 0.83 | 0.04 | 0.05 | 0.83 | 2.36 × 105 | 2.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jia, Q.; Liu, Y.; Chen, D.; Fang, Z.; Liu, Y.; Li, S.; Hu, B.; Wang, C.; Chen, H. Different Structures of Arabinoxylan Hydrolysates Alleviated Caco-2 Cell Barrier Damage by Regulating the TLRs/MyD88/NF-κB Pathway. Foods 2022, 11, 3535. https://doi.org/10.3390/foods11213535
Li J, Jia Q, Liu Y, Chen D, Fang Z, Liu Y, Li S, Hu B, Wang C, Chen H. Different Structures of Arabinoxylan Hydrolysates Alleviated Caco-2 Cell Barrier Damage by Regulating the TLRs/MyD88/NF-κB Pathway. Foods. 2022; 11(21):3535. https://doi.org/10.3390/foods11213535
Chicago/Turabian StyleLi, Jingwen, Qi Jia, Ying Liu, Daiwen Chen, Zhengfeng Fang, Yuntao Liu, Shanshan Li, Bin Hu, Caixia Wang, and Hong Chen. 2022. "Different Structures of Arabinoxylan Hydrolysates Alleviated Caco-2 Cell Barrier Damage by Regulating the TLRs/MyD88/NF-κB Pathway" Foods 11, no. 21: 3535. https://doi.org/10.3390/foods11213535
APA StyleLi, J., Jia, Q., Liu, Y., Chen, D., Fang, Z., Liu, Y., Li, S., Hu, B., Wang, C., & Chen, H. (2022). Different Structures of Arabinoxylan Hydrolysates Alleviated Caco-2 Cell Barrier Damage by Regulating the TLRs/MyD88/NF-κB Pathway. Foods, 11(21), 3535. https://doi.org/10.3390/foods11213535